The rapid expansion of large language models’ (LLMs) capabilities—including web search, code execution, data…
Category: 7. Science
-
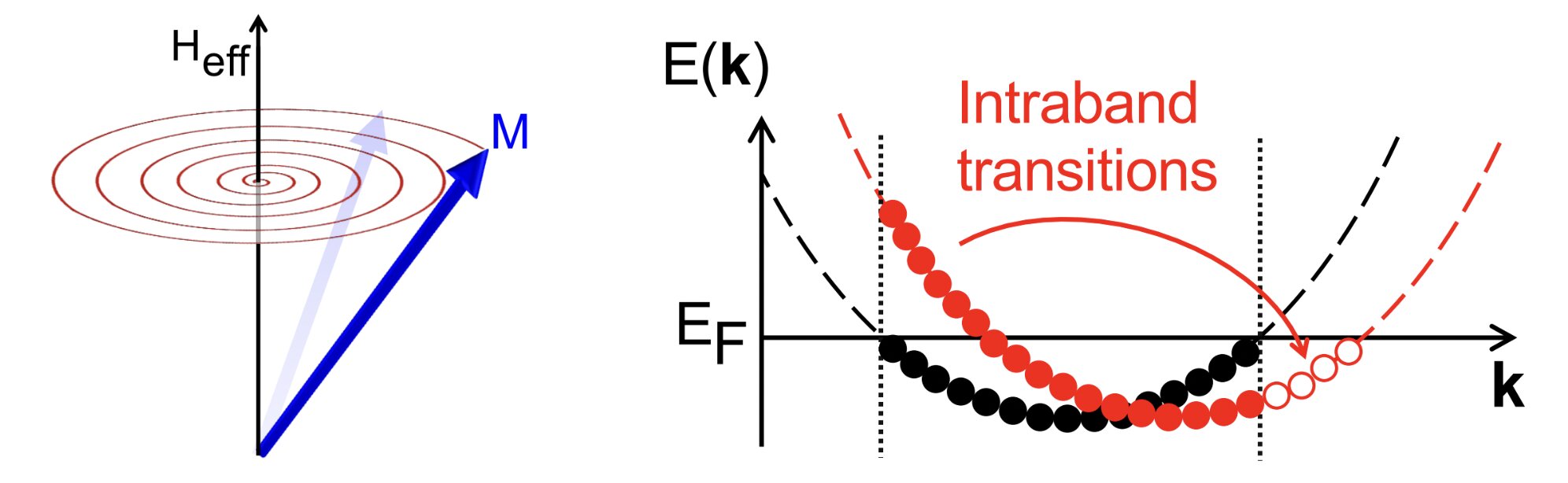
Mirror symmetry prompts ultralow magnetic damping in 2D van der Waals ferromagnets
Left panel: Schematic illustration of magnetic damping. Right panel: Sketch of intraband transitions. Equilibrium occupation of an energy band (black) evolves out of… Continue Reading
-

Water Causes Rock To Shift On Matterhorn
On the Matterhorn, an international research team led by Jan Beutel from the Department of Computer Science shows how meltwater in permafrost can lead to rock slope instability. A rock pillar collapsed there in 2023 after years of water…
Continue Reading
-

Scientists shrink 3-D printing so it can work inside cells
3-D printing: The creation of a three-dimensional object with a machine that follows instructions from a computer program. The computer tells the printer where to lay down successive layers of some raw material, which can be plastic, metals,…
Continue Reading
-
With two launches in the books, Blue Origin announces even more powerful New Glenn is coming – Phys.org
- With two launches in the books, Blue Origin announces even more powerful New Glenn is coming Phys.org
- New Glenn Update Blue Origin
- Blue Origin planning next New Glenn flight for early next year SpaceNews
- NASA, Blue Origin Launch Two Spacecraft to…
Continue Reading
-

Colorful life on exoplanets might be lurking in clouds
View larger. | Artist’s concept of a rocky planet like Earth with colorful microbes in its clouds. The microorganisms have biopigments similar to ones on Earth. New research shows that telescopes could detect signs of such colorful life on Continue Reading
-

How long does it take to walk 2 miles? A running coach’s pace-by-pace estimates
Whether you’re planing a quick workout, tracking your daily steps or just curious how far an evening stroll will take you, understanding how long it takes to walk two miles can help you plan your exercise routine and identify the right intensity…
Continue Reading
-

Mysterious galaxy trapped in ‘the void’ keeps churning out stars without fuel. Scientists are stumped.
Scientists are puzzled by an”impossible” galaxy that doesn’t appear to have the fuel it needs to be growing.
The dwarf galaxy, NGC 6789, is located approximately 12 million light-years from Earth, in an empty region known as the Local Void, and…
Continue Reading
-

Can fish feel pain? That may be the wrong question.
What must it feel like to be a fish — to glide weightlessly through the sea, to draw breath from water, to be (if one is lucky) oblivious to the parched terrestrial world above?
Maybe you suspect there isn’t much to fish — and you could…
Continue Reading
-

James Webb Space Telescope spots a gassy baby galaxy throwing a tantrum in the early universe
A baby galaxy is throwing one heck of a tantrum, and it’s shaking up our understanding of the earliest galaxies.
Recently, an international team of astronomers used the James Webb Space Telescope (JWST) to uncover a bright, young galaxy in the…
Continue Reading