In the distant reaches of the solar system are many icy objects that resemble snowmen — pairs of conjoined spheres. Now, a new study reveals the simple way in which these mysterious objects might form.
Beyond the orbit of Neptune lie icy…
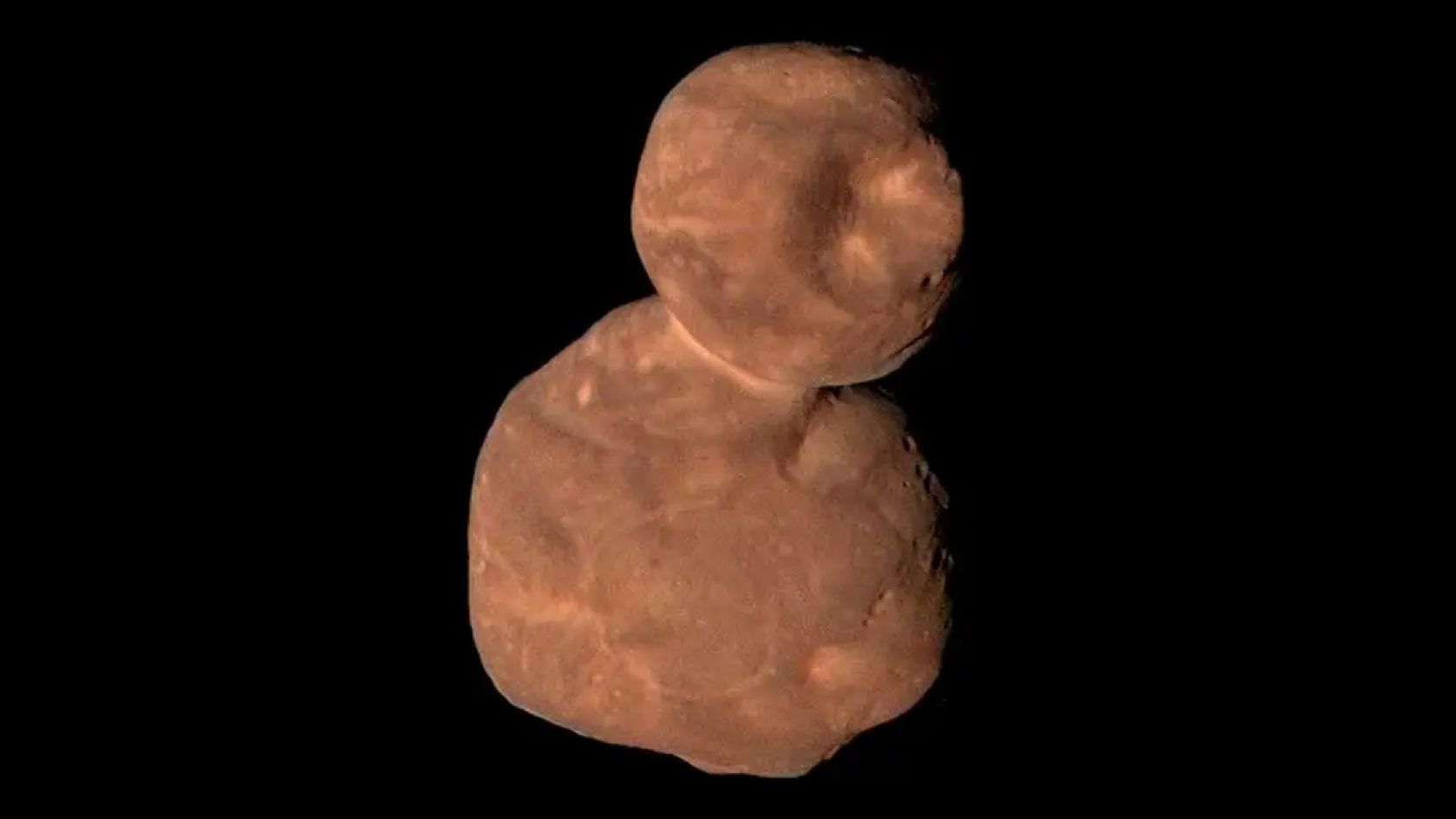
In the distant reaches of the solar system are many icy objects that resemble snowmen — pairs of conjoined spheres. Now, a new study reveals the simple way in which these mysterious objects might form.
Beyond the orbit of Neptune lie icy…

Terms
While we only use edited and approved content for Azthena
answers, it may on occasions provide incorrect responses.
Please confirm any data provided with the related suppliers or
…

The Pakistan Meteorological Department has announced that the year’s first total lunar eclipse will occur on March 3 and will be partially observable in several cities across Pakistan.
Commonly referred to as a “Blood Moon,” this celestial…

Astronomers have unveiled an intriguing discovery: the largest image ever…

There is a corner of Antarctica that looks like something out of a Cronenberg movie. It lies in the McMurdo Dry Valleys, an immense frozen wilderness where, periodically, a stream of crimson-red liquid suddenly gushes from the dazzling white…

Terms
While we only use edited and approved content for Azthena
answers, it may on occasions provide incorrect responses.
Please confirm any data provided with the related suppliers or
…
…

This weekend is the last chance to see any of February’s much-hyped “planet parade” or “planetary parade” before it completely fades from view. While six planets are technically above the horizon after sunset, the lineup is…

NASA on Wednesday was rolling its towering Moon rocket back to its hangar for repairs, after windows for liftoff were pushed back over technical issues.
The US space agency said it would roll its 322-foot (98-meter) SLS rocket off the launchpad…

Dawn on a still morning is a majestic spectacle, as sunlight spills silently across the landscape and the Earth gradually emerges from darkness. Sunrise has inspired countless pieces of music striving to express this soundless experience in…