Category: 7. Science
-
Prehistoric tool made from elephant bone is the oldest discovered in Europe – Phys.org
- Prehistoric tool made from elephant bone is the oldest discovered in Europe Phys.org
- News – Could Toolmaking Abilities Be Linked to Speech? Archaeology Magazine
- In the 1990s, scientists dug up a 500,000-year-old tool in England. They just figured…
Continue Reading
-

Watch a robot swarm “bloom” like a garden
Researchers at Princeton University have built a swarm of interconnected mini-robots that “bloom” like flowers in response to changing light levels in an office. According to their new…
Continue Reading
-
Study Suggests A Pathway For Life-sustaining Conditions In Europa’s Ocean – astrobiology.com
- Study Suggests A Pathway For Life-sustaining Conditions In Europa’s Ocean astrobiology.com
- Study suggests pathway for life in Europa’s ocean WSU Insider
- Europa ice delamination may deliver nutrients to hidden ocean Space Daily
- This…
Continue Reading
-

Stunning Footage Shows Space Station Drifting Through Aurora’s Dazzling Lights
Earlier this week, the Sun unleashed a powerful X-class solar flare, a major burst of electromagnetically charged particles that lit up the Earth’s night sky as they entered our planet’s atmosphere.
The effect was stunning: a dazzling…
Continue Reading
-

New sungrazing comet officially named. See maps here!
This is the new sungrazing comet C/2026 A1 (MAPS). Image via Gerald Rhemann and Michael Jäger. Used with permission. New sungrazing comet inbound
There’s a new comet headed into the inner solar system, and it will make an extremely close pass…
Continue Reading
-
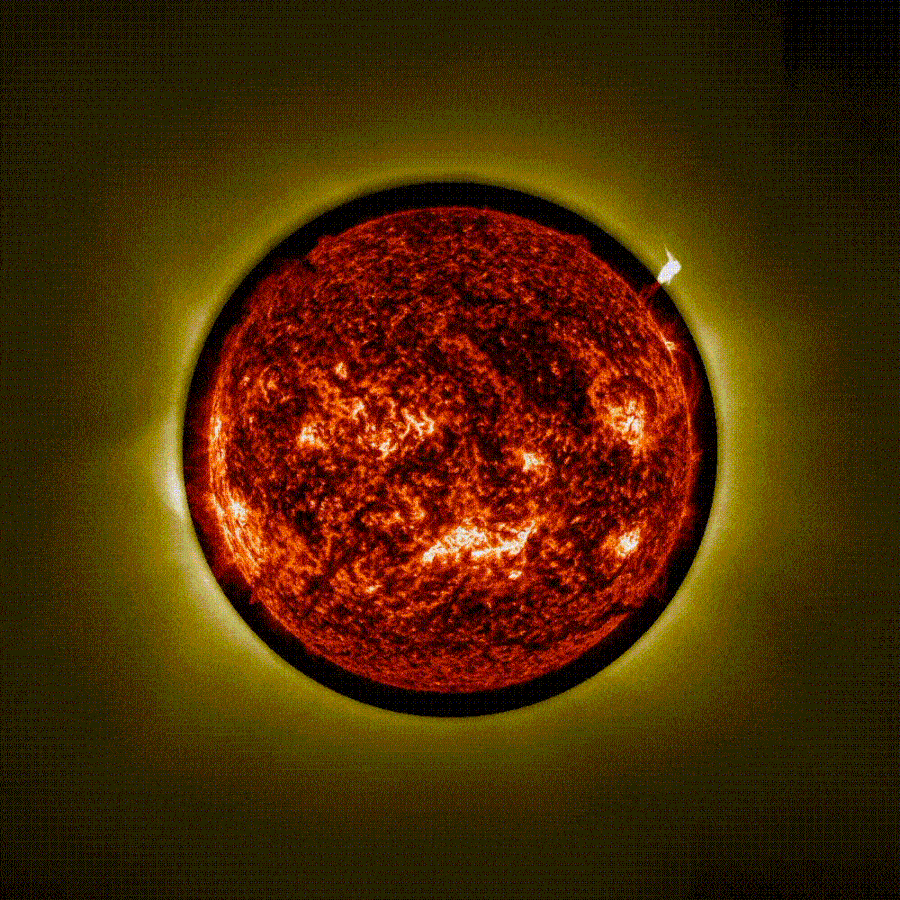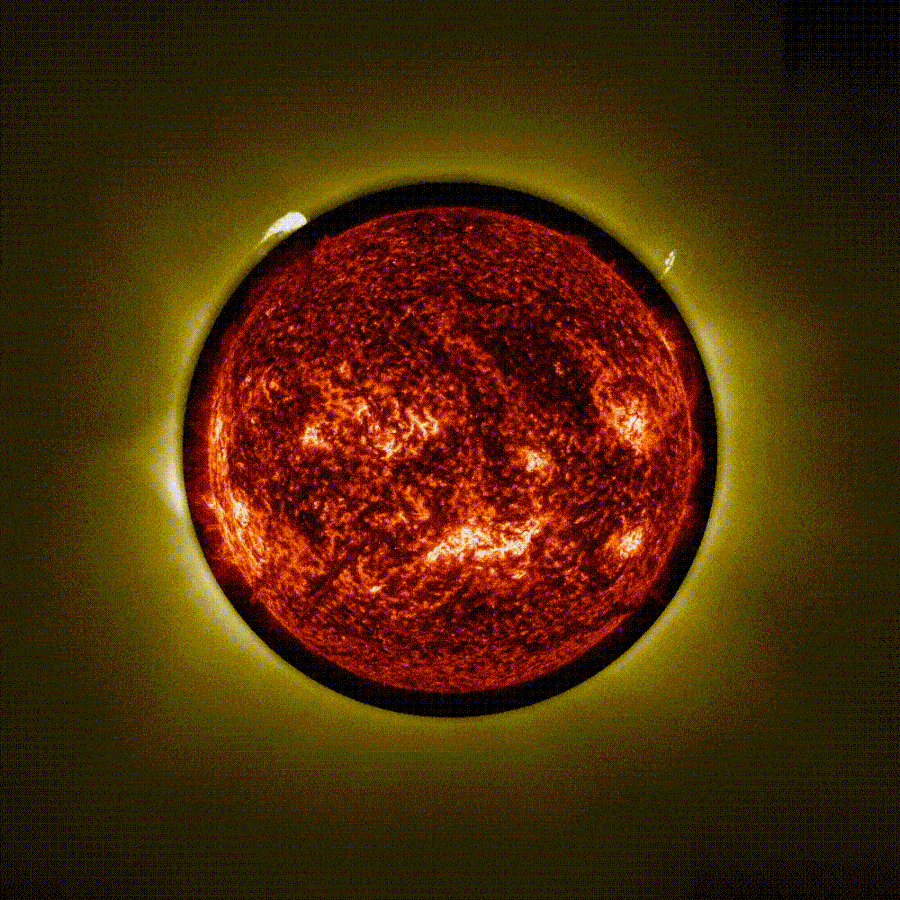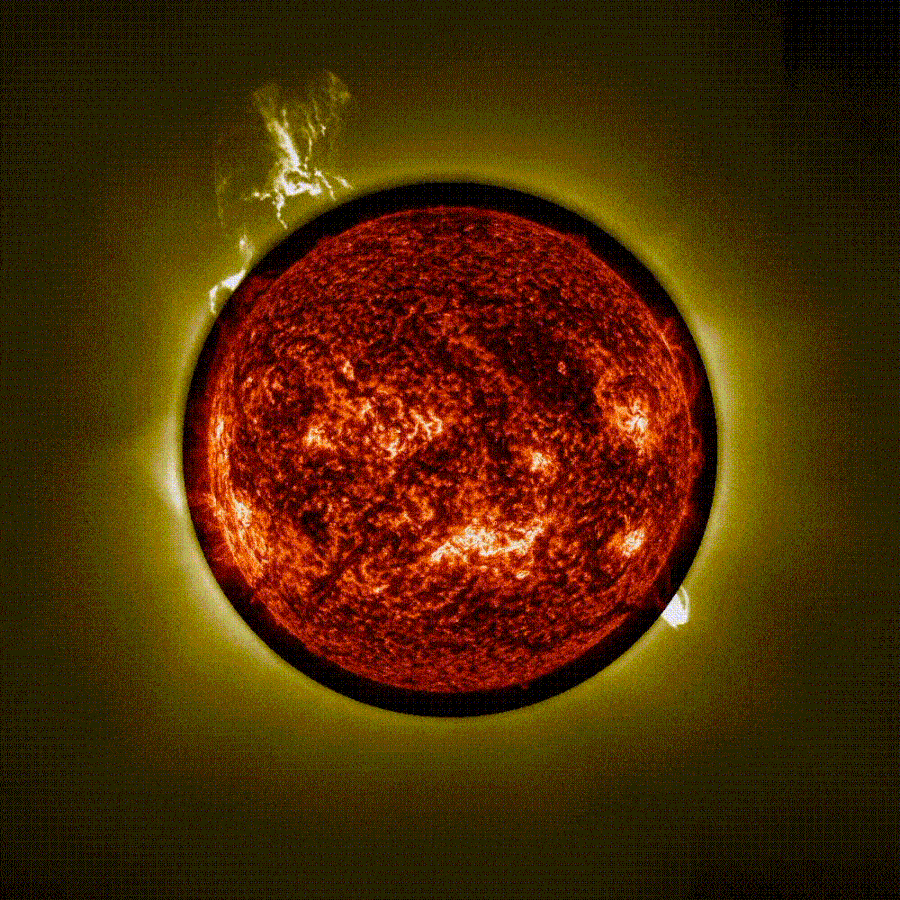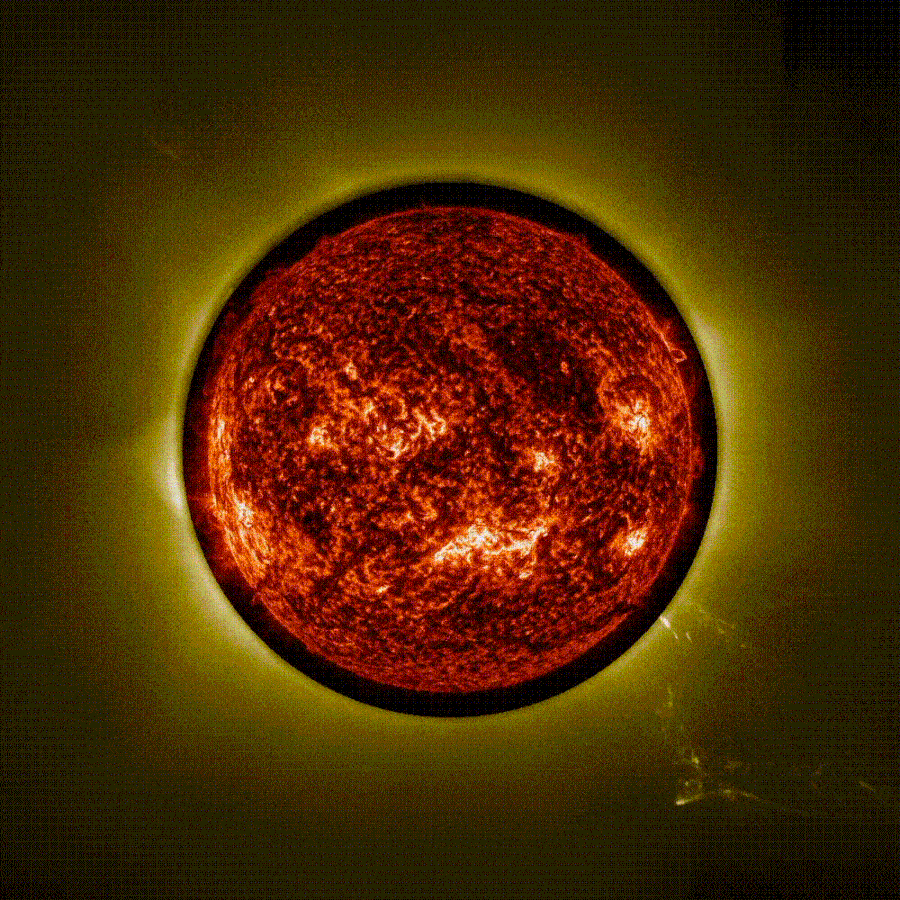
Stunning time-lapse video captured using ‘artificial eclipse’ shows 3 massive eruptions on the sun
The European Space Agency (ESA) has released a stunning time-lapse of a trio of solar eruptions exploding into space from the sun during an “artificial eclipse.” The unique footage, captured by the newly operational Proba-3 mission, could help…
Continue Reading
-

Shifting the PPARγ conformational ensemble toward a transcriptionally repressive state improves covalent inhibitor efficacy
Peroxisome proliferator-activated receptor gamma (PPARγ) regulates gene expression programs that influence cellular differentiation and insulin sensitization in response to ligand binding. The discovery in 1995 that PPARγ is a molecular…
Continue Reading
-

Enormous freshwater reservoir discovered off the East Coast may be 20,000 years old and big enough to supply NYC for 800 years
A giant reservoir of “secret” fresh water off the East Coast that could potentially supply a city the size of New York City for 800 years may have formed during the last ice age, when the region was covered in glaciers, researchers…
Continue Reading

