Nasa is preparing to roll out its most powerful rocket yet before a mission to send astronauts around the moon and back again for the first time in more than 50 years.
The Artemis II mission is scheduled to launch from Kennedy Space Center in…

Nasa is preparing to roll out its most powerful rocket yet before a mission to send astronauts around the moon and back again for the first time in more than 50 years.
The Artemis II mission is scheduled to launch from Kennedy Space Center in…
MELBOURNE, Jan. 17 (Xinhua) — An international team of scientists has discovered a new way immune cells recognize molecules in the body, challenging a long-standing view of how the immune system works.
The study, published in Nature…
MELBOURNE, Jan. 17 (Xinhua) — An international team of scientists has discovered a new way immune cells recognize molecules in the body, challenging a long-standing view of how the immune system works.
The study, published in Nature…
Calver, A. R. et al. Molecular cloning and characterisation of a novel GABAB-related G-protein coupled receptor. Brain Res. Mol. Brain Res. 110 (2), 305–317 (2003).
Charles, K. J. et al….

SpaceX launched its first national security mission of the year on Friday night (Jan. 16), sending a batch of spy satellites aloft from California.
A Falcon 9 rocket lifted off from California’s Vandenberg Space Force Base Friday at 11:39 p.m….
An international team of scientists, led by Nanyang Technological University, Singapore (NTU Singapore), has discovered a new way that could speed up the healing of chronic wounds infected by antibiotic-resistant…
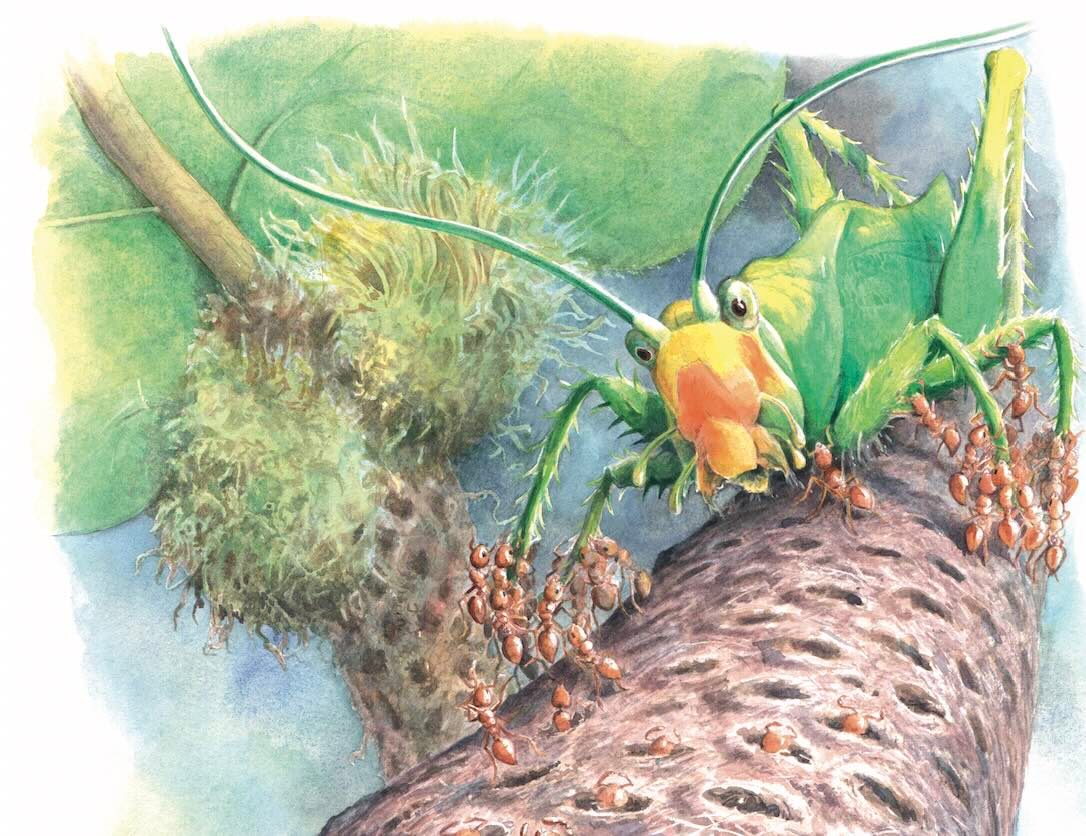
Somewhere in the Amazonian understorey, beneath a great forest canopy, a cricket leaps on to the stem of a shrub. It’s the last leap it will ever make.
It never gets to flex its legs again. It’s stuck, like fluff to Velcro. Dozens of tiny…
BYLINE: Leigh Hataway
Newswise — Ever see a basset hound and find yourself wanting to (gently) grab its long, floppy ears and give them a little waggle?
The cute aggression…