56 million years ago, the Earth was already warm. ‘As a result, there was a lot of vegetation, even at high latitudes. That means that a lot of carbon was stored in, for example, vast coniferous forests.’ Biologist Mei Nelissen is…
Category: 7. Science
-

What the first medical evacuation from the International Space Station tells us about healthcare in space
This article was originally published at The Conversation. The publication contributed the article to Space.com’s Expert Voices: Op-Ed & Insights.
For the first time in 25 years of continuous crewed operations, an astronaut has been medically…
Continue Reading
-

How to find 4 legendary spacecraft in January’s night sky
We live in the age of the spacefaring robot, wherein far-flung probes brave the harsh environment of interplanetary space to beam gorgeous images and invaluable scientific data back to Earth to broaden our understanding of the solar system that…
Continue Reading
-

The race to build a super-large ground telescope is likely down to two competitors
I have been writing about the Giant Magellan Telescope for a long time. Nearly two decades ago, for example, I wrote that time was “running out” in the race to build the next great…
Continue Reading
-
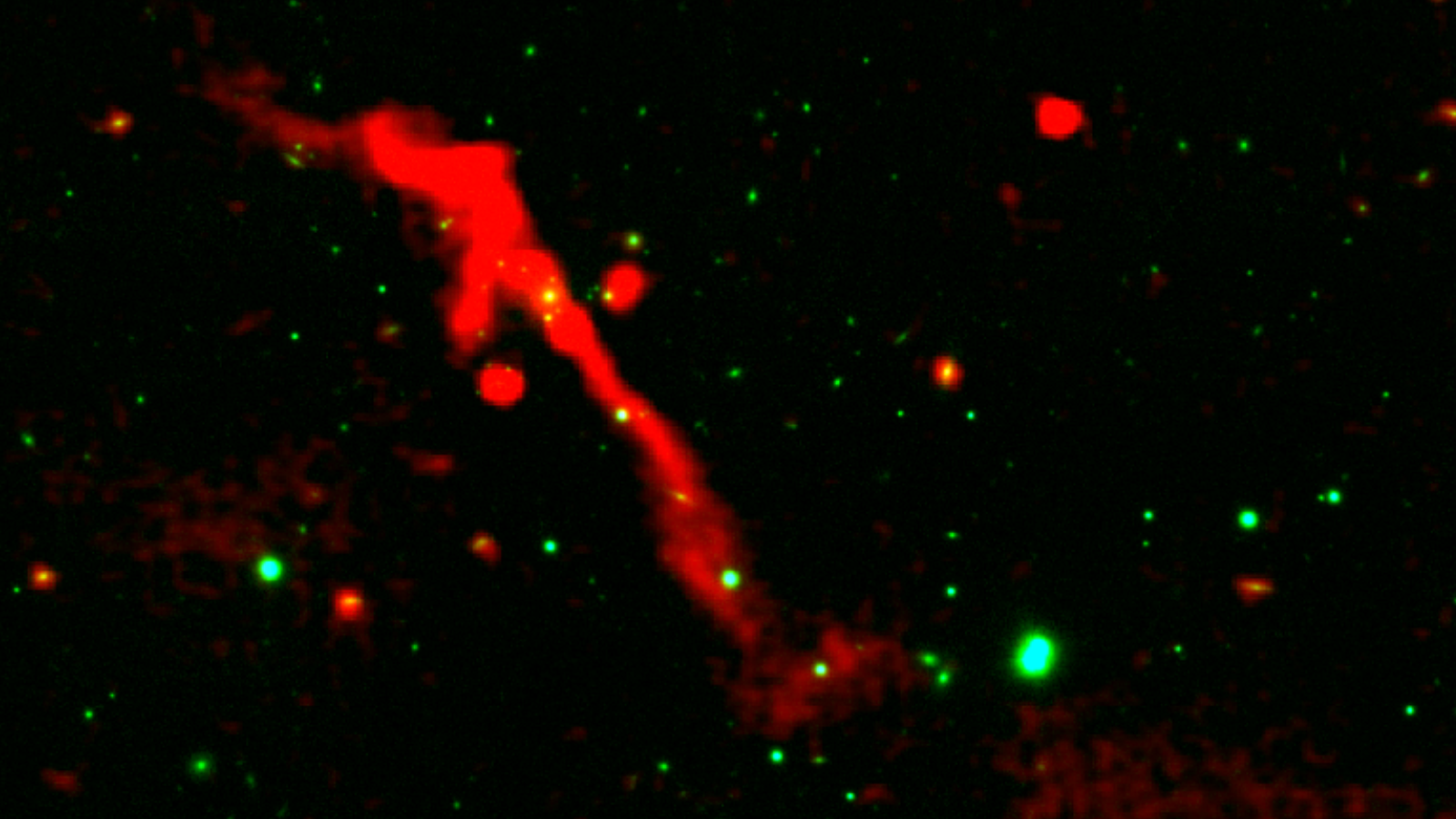
Reborn black hole seen erupting across 1 million light-years of space like a cosmic volcano
Astronomers have discovered a once-dormant supermassive black hole springing back to life in a very dramatic and spectacular fashion, acting as a “cosmic volcano” blasting out an eruption that stretched out for 1 million light-years. The…
Continue Reading
-

The Last Common Ancestor of Humans and Neanderthals Is Found, in Morocco
We are eternally fascinated by the mystery of our origins. Dust and a divine spark satisfied many until the advent of evolution theory and paleontology, which generated a broad consensus that our species originated in Africa. But doubts had…
Continue Reading
-

Austrian Cow Displays First Tool Use in Cattle
In 1982, cartoonist Gary Larson published a now-iconic Far Side comic entitled Cow Tools. In it, a cow stands proudly beside a jumble of bizarre, useless objects that are “tools” in name only. The joke hinged on a simple assumption:…
Continue Reading
-

Back-scratching bovine leads scientists to reassess intelligence of cows | Animal behaviour
Scientists have been forced to rethink the intelligence of cattle after an Austrian cow named Veronika displayed an impressive – and until now undocumented – knack for tool use.
Witgar Wiegele, an organic farmer and baker from a small town in…
Continue Reading
-
What the first medical evacuation from the International Space Station tells us about health care in space – Phys.org
- What the first medical evacuation from the International Space Station tells us about health care in space Phys.org
- NASA’s SpaceX Crew-11 Mission Returns, Splashes Down off California NASA (.gov)
- ISS SOS: The plan to leave a doomed space…
Continue Reading

