“Even limited use of veins appears to diminish the long-term benefits of an all-arterial approach,” says Alistair Royse.
Relying entirely on arteries as a source for grafts in CABG appears to impart better survival for patients…
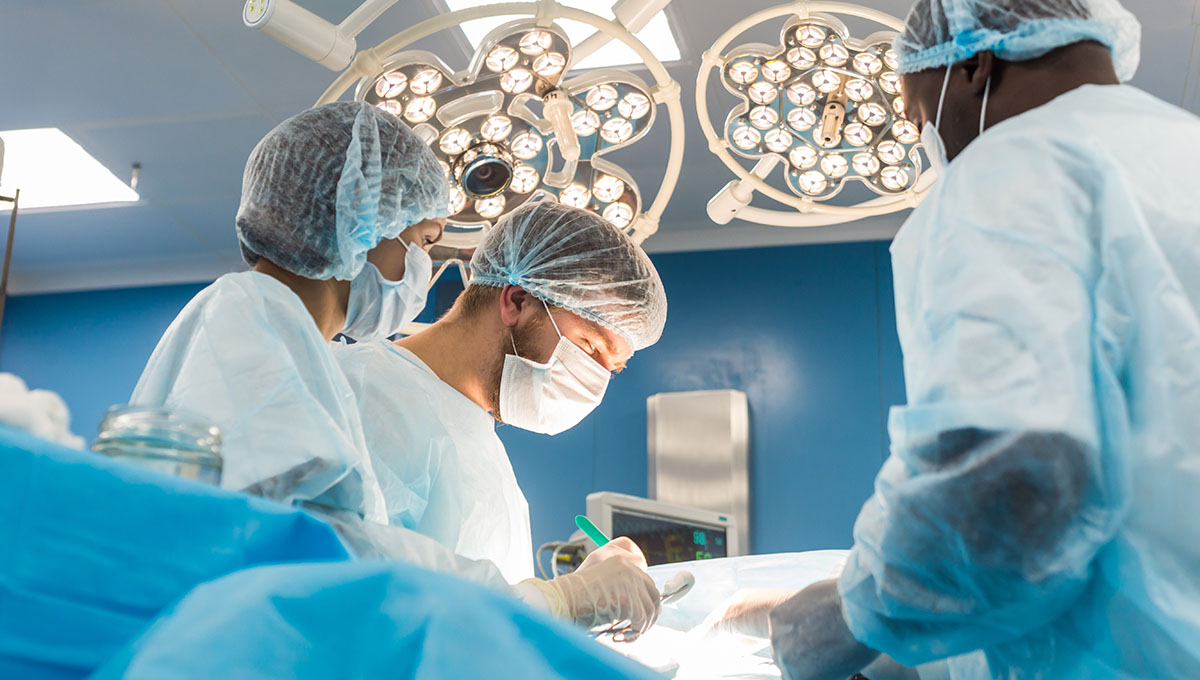
Relying entirely on arteries as a source for grafts in CABG appears to impart better survival for patients…

Benzinga and Yahoo Finance LLC may earn commission or revenue on some items through the links below.
Bitcoin’s (CRYPTO: BTC) flash crash late Friday, which happened outside of regular trading hours, has reignited debate about the operating hours of spot BTC exchange-traded funds, particularly the iShares Bitcoin Trust ETF (NASDAQ:IBIT).
The apex cryptocurrency collapsed from $116,000 to under $110,000 within minutes after President Donald Trump threatened 100% tariffs on China in response to “aggressive stance” on export controls.
The sudden spike caught traders off-guard, triggering over $19 billion in liquidations, the largest single-day wipeout in cryptocurrency history.”
Trending: Missed Nvidia and Tesla? RAD Intel Could Be the Next AI Powerhouse — Just $0.81 a Share
The crash fueled concerns about the lack of 24/7 trading of BTC exchange-traded funds, which have become a popular vehicle for institutional investors, Forbes reported, quoting Tommy Doyle, global head of client management at Xapo Bank.
“The extreme volatility in the price of Bitcoin overnight highlights why institutional investors increasingly view access to 24/7 liquidity as essential to prudent risk management,” Doyle said.
Notably, these ETFs, including the nearly $100 billion IBIT, are bound by stock trading hours, which prevents investors from responding to weekend price shifts.
“Whilst Bitcoin ETFs remain bound by traditional market trading hours, institutional investors with direct bitcoin accounts can continue to access liquidity and risk manage their bitcoin exposure throughout the weekend, especially relevant amid recent seismic price moves,” Doyle told Forbes.
See Also: Accredited Investors Can Now Tap Into the $36 Trillion Home Equity Market — Without Buying a Single Property
It’s worth noting that Robinhood allows trading from 8 p.m. ET on Sunday until 8 p.m. ET on Friday, with some restrictions.
BlackRock’s IBIT ETF is the largest cryptocurrency-based investment fund currently in operation, with assets under management totaling almost $94 billion, according to SoSo Value.
Overall, BTC ETFs reported net inflows exceeding $2.70 billion for the week ended Oct.10.
Photo Courtesy: Arsenii Palivoda on Shutterstock.com
Trending Now:

You may have missed it, but the internet had a collective meltdown about the brand-new iPhone 17 Pro, Pro Max, and iPhone Air demo units looking scratched up in stores. We finally have an answer. And no, the phones are not, in fact, made of…

COPENHAGEN, Denmark—Almost half of patients with no history of arrhythmias who undergo CABG for three-vessel or…

Breast tumor trends are similar between women in Ghana and Black women in the U.S., suggest findings published October 13 in JAMA Network Open.
Researchers led by Brittny Davis Lynn, PhD, from the National Cancer Institute in Rockville, MD,…

Donald Trump has vowed to use the power of his presidency to ensure that Israel recognises it has achieved “all that it can by force of arms”, and begin an age of cooperation in the Middle East that may ultimately extend as far as peace with…
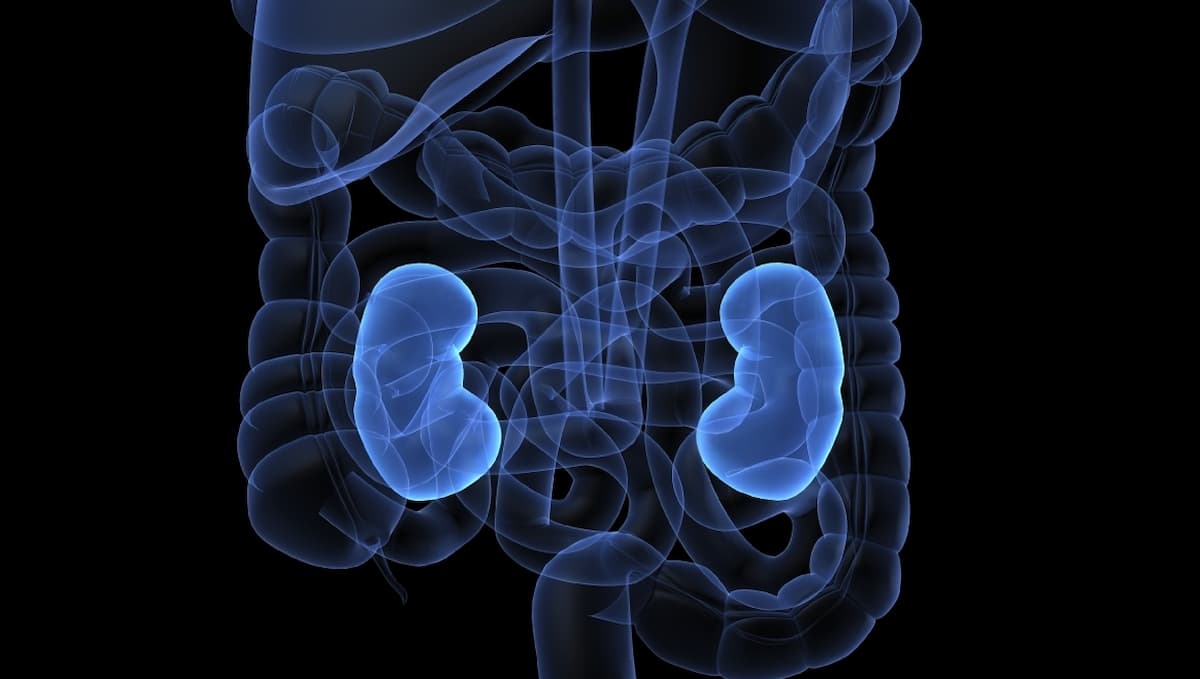
Combining fruquintinib (Fruzaqla) with sintilimab (Tyvyt) significantly improved progression-free survival (PFS) vs axitinib (Inlyta) or everolimus (Afinitor) monotherapy among patients with locally advanced or metastatic renal cell carcinoma (RCC), according to a press release on findings from the
With a median follow-up of 16.6 months and a cutoff date of February 17, 2025, data from the phase 3 portion of the trial revealed a median PFS of 22.2 months in the fruquintinib arm and 6.9 months in the comparator arm (stratified HR, 0.373; P <.0001). Additionally, the objective response rate (ORR) was 60.5% vs 24.3% (OR, 4.622; P <.0001), with a median duration of response (DOR) of 23.7 months and 11.3 months in each arm.
Data related to overall survival (OS) were still maturing at the time of analysis. Investigators noted efficacy benefits with the fruquintinib combination across all prognostic risk subgroups based on the International mRCC Database Consortium (IMDC) guidelines.
Treatment with fruquintinib plus sintilimab appeared to be tolerable and comparable with prior reports of each agent. Grade 3 or higher treatment-emergent adverse effects (TEAEs) occurred in 71.4% of the experimental arm and 58.8% of the comparator arm.
Investigators will present detailed results from the FRUSICA-2 trial at the
“The FRUSICA-2 trial results provide compelling evidence that fruquintinib and sintilimab may offer a valuable new treatment option for patients with advanced [RCC],” co-leading principal trial investigator Dingwei Ye, MD, PhD, a professor at Fudan University Shanghai Cancer Center, stated in the press release.1 “These findings show the combination’s potential to address a critical unmet need for this patient population, delivering consistent benefits across varied patient profiles and prognostic risk groups.”
According to the press release, China’s National Medical Products Administration is currently reviewing a new drug application for fruquintinib plus sintilimab as a treatment for those with previously treated metastatic or locally advanced RCC based on data from the FRUSCIA-2 trial.
Investigators of the open-label, active-controlled, registration FRUSICA-2 trial assessed the safety and efficacy of fruquintinib plus sintilimab vs monotherapy with axitinib or everolimus among 234 patients with second-line advanced RCC. In the combination arm, patients received fruquintinib at 5 mg orally each day in a 2-week-on, 1-week-off schedule every 3 weeks per cycle plus sintilimab at 200 mg intravenously every 3 weeks.2
The trial’s primary end points included PFS in part 1 and ORR in part 2. Secondary end points included the disease control rate, DOR, time to response, OS, safety, and quality of life.
Patients 18 to 75 years old with histologically or cytologically confirmed RCC, locally advanced or metastatic disease, and progression on or after prior frontline treatment with a VEGF-directed tyrosine kinase inhibitor were eligible for enrollment on the trial. Other eligibility criteria included having at least 1 measurable lesion per RECIST v1.1 guidelines, an ECOG performance status of 0 or 1, and adequate organ function.
Those with prior receipt of approved systemic anti-cancer therapy within 2 weeks of beginning study treatment, active autoimmune or inflammatory diseases, or known central nervous system metastases were ineligible to enroll on the trial. Patients were also unsuitable for study entry if they had a history of pneumonitis requiring corticosteroid therapy, a history of or current interstitial lung disease, or current active pulmonary infection.
“The FRUSICA-2 study suggests that fruquintinib and sintilimab could play a meaningful role in shaping second-line treatment strategies for advanced [RCC],” co-leading principal investigator Zhisong He, a professor at Peking University First Hospital, said in the press release.1 “These results point to the combination’s potential to enhance clinical outcomes, providing a new option for managing this challenging disease.”
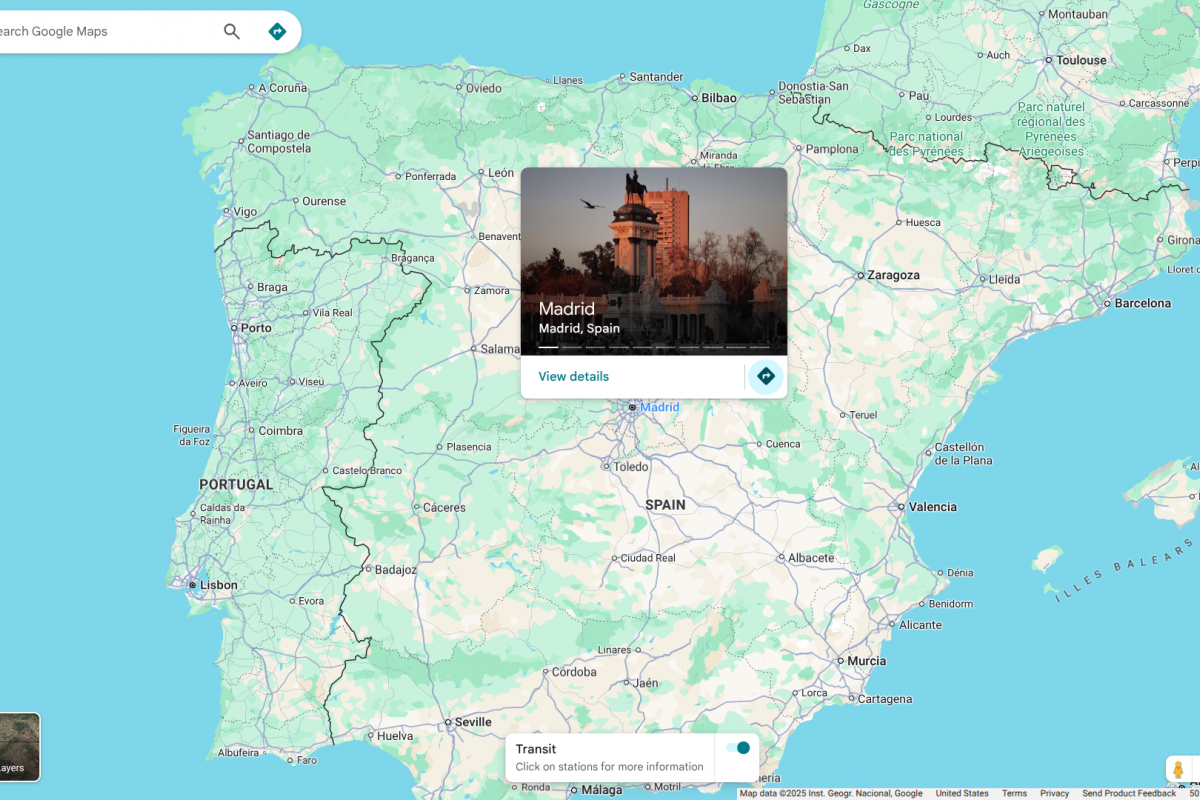
According to Spanish media, a man residing in the Community of Madrid is the first Mpox clade 1b case of autochthonous (local) infection of the most contagious strain of the sexually transmitted virus.
The Madrid…