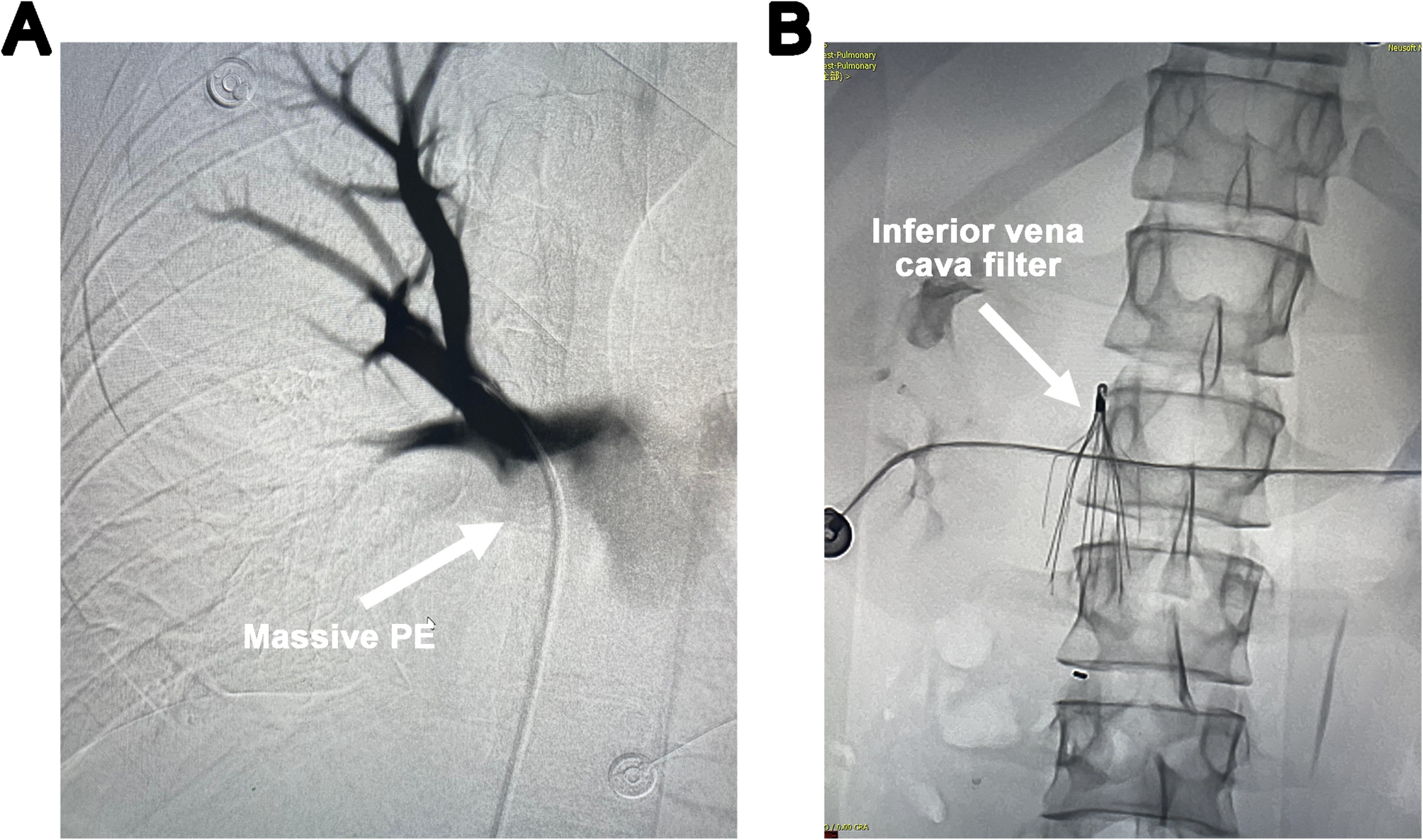
An 18-year-old male patient, a Moroccan international student in China, presented to the emergency department of Union Hospital, Tongji Medical College, Huazhong University of Science and Technology, with a chief complaint of persistent upper…
Blog
-

These teams that have qualified for the IFAF Flag Football World Championships 2026
The fields are now set for the women’s and men’s competitions at the 2026 IFAF Flag Football World Championships.
Following the conclusion of the Asia-Oceania Flag Football Championships on Sunday (26 October), the final continental competition…
Continue Reading
-

AI Helps Decode The Epigenetic ‘off-switch’ In An Ugly Plant That Lives For 3,000 Years
We all age, and for decades, science has been looking for a way to slow that clock. Much of that research is now focused on epigenetics—a system of “software” that runs on our genetic “hardware,” telling genes when to turn on and off.…
Continue Reading
-

Sotheby’s Records Surrealist Records in Paris—and More Art News
The Headlines
SUSPECTED LOUVRE THIEVES ARRESTED. Two suspects have been detained in connection with the theft of jewels, including pieces once belonging to Emperor Napoleon III and Empress Eugénie, from the Louvre on October 19….
Continue Reading
-

Eli Lilly buys Adverum in eye disease gene therapy punt
As part of the deal, Eli Lilly will acquire Adverum’s lead candidate ixo-vec, an intravitreal gene therapy for wAMD treatment. Credit: Tada Images via Shutterstock.com. 
Eli Lilly has agreed to acquire eye disease specialist Adverum Biotechnologies, bucking a recent trend of big pharma companies deciding to steer clear of the cell and gene therapy sector.
Eli Lilly has offered Adverum $3.56 per share in cash, including an additional $8.91 in milestone payments. The latter depends on US approval of the biotech’s lead gene therapy candidate, ixo-vec, within seven years and achieving more than $1bn in annual global sales within ten years. This brings the total consideration to $12.47 a share, valuing the deal at a possible $261.7m.
Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
Find out more
The share offer agreed on 24 October reflects a nearly 15% discount from the $4.18 closing price on 23 October.
For Adverum, the potential buyout from Eli Lilly provides financial respite. The biotech has been struggling for cash in recent times – holding $44.4m to its name in July 2025. The lack of capital had increased jeopardy for ixo-vec, an intravitreal gene therapy that advanced into a Phase III trial (NCT06856577) for the treatment of wet age-related macular degeneration (wAMD) in March 2025.
Indeed, Eli Lilly stated that without a $65m loan given to Adverum to continue ongoing clinical trials, the biotech would only be able to finance itself through October before having to wind down operations.
Despite having to help fund ixo-vec’s development, which has been granted fast track and regenerative medicine advanced therapy (RMAT) designations by the US Food and Drug Administration (FDA), Eli Lilly could use the candidate to enter the lucrative wAMD market. The AMD sector, which also includes the dry form, is expected to reach $27.5bn across 7MM by 2031 (7MM: US, France, Germany, Italy, Spain, UK, and Japan), according to GlobalData analysis.
There is no gene therapy approved with a wAMD indication, with current treatments working via the anti–vascular endothelial growth factor (VEGF) mechanism, such as Regeneron’s blockbuster Eylea (aflibercept). The therapy is administered every four weeks for the first five months, followed by a single injection every two months. For Eli Lilly’s soon-to-be acquired ixo-vec, this could offer patients a one-and-done treatment.
Lilly molecule discovery group vice-president Andrew Adams said: “Ixo-vec has the potential to transform wAMD treatment from a paradigm of chronic care with repeated intravitreal injections to a convenient one-time therapy.”
Adverum CEO Laurent Fischer: “[Lilly’s] scientific depth and global reach offer the opportunity to accelerate our vision to deliver a transformative one-and-done therapy that can potentially restore and preserve vision for millions of patients living with wAMD.”
Lilly bucks big pharma trend
This is not the first time in 2025 that Eli Lilly has swooped in to rescue a cash-strapped biotech specialising in gene therapies. In April, the big pharma signed a licensing deal worth up to $1.4bn for Sangamo Therapeutics’ neurology-targeting gene therapy.
However, Lilly’s recent deals, which includes a $1.3bn acquisition of RNA-based gene therapy developer Rznomics in May 2025, goes against the grain of big pharma generally opting to retreat from the cell and gene therapy sector.
Earlier this month, Galapagos wound down its cell and gene therapy division after failing to sell the unit. Japanese pharma Takeda also abandoned its cell therapy research, pivoting instead towards small molecules, biologics and antibody-drug conjugates (ADCs).
In addition, Gilead Sciences’ Kite Pharma terminated its cell therapy collaboration with Shoreline in September 2025, ending a research partnership valued at $2.3bn.
Cell & Gene Therapy coverage on Pharmaceutical Technology is supported by Cytiva.
Editorial content is independently produced and follows the highest standards of journalistic integrity. Topic sponsors are not involved in the creation of editorial content.
Continue Reading
-

Bitcoin faces a new civil war over how its blockchain should be used
I recently had the pleasure of visiting the lovely mountain town of Lugano, Switzerland, whose appeal lies in that it is basically Italy but administered by the Swiss. That’s according to Tether CEO Paolo Ardoino, one of the prime backers of Plan B, a Bitcoin conference where I hosted a discussion on the growing trend of nation states embracing the original cryptocurrency.
The event had an upbeat vibe—not surprising since everyone there worshipped Bitcoin—but it was also clear there was trouble in paradise. It turns out there is a growing schism over Bitcoin’s codebase, and whether it should be modified to permit the blockchain to include more non-financial data.
The notion of including data unrelated to Bitcoin transactions is hardly new and, indeed, the very first block on the blockchain includes a reference to a newspaper headline about bank bailouts. Now, though, Bitcoin’s biggest and most influential group of coders, known as Core, are planning to tweak their software in order to significantly lift the restrictions on how much non-payment information can be included in a block.
For the Core crowd, this is a simple and pragmatic way to promote new uses for Bitcoin and, in the process, drum up extra fees for miners at a time when the blockchain’s lottery payment is 3.125 Bitcoins, and set to halve again in 2028. A fast-growing rival faction, though, wants nothing to do with the scheme and is promoting a Bitcoin client software of its own called Knots.
That faction’s software is led by an influential Bitcoin developer, who is a devout Catholic and reportedly named it Knots after the “whip of knots” Jesus used to drive money changers from a temple. According to a lawyer I spoke with on the Knots side, the software is necessary to protect the blockchain from what he decried as spammers and “scam adjacency” projects that promote things like Bitcoin NFTs.
If you’ve encountered Bitcoiners in person or online, you’re aware they’re not known for their tact. That is true of prominent figures from Bitcoin’s early days who have been denouncing each other on stage in Lugano and on X. These high profile partisans include Peter Todd and Jameson Lopp for the Core faction, and Nick Szabo and Luke Dashjr for the rival Knots sect.
This latest schism (you can read a helpful breakdown here) hearkens back to the Bitcoin block size wars that raged from 2015 to 2017, and ultimately saw the “small blockers”—who favored keeping Bitcoin blocks at 1MB—prevail over rivals who claimed boosting the blocks to 2MB or more would be more commercially viable. That fight produced bad blood that has lasted to this day.
In the current fight, Knots is still the smaller faction, but has already become the client of choice for over 20% of Bitcoin node operators. Its growing popularity lies not only in Knots’ position on expanding the blockchain, but from a perception that the Core crowd has grown arrogant and out-of-touch with Bitcoin’s core values. The Core folks, meanwhile, dismiss the Knots faction as lying trouble-makers.
I lack the authority to weigh in on much of this, other than to observe that this latest battle for the soul of Bitcoin reinforces what I’ve said for years: Bitcoin is a marvelous technology, but also a religion. And with any religion, there will be divisions between old-line believers and more modern adherents. Happily for the crowd in Lugano, there was a moment of unity that came with the unveiling of a restored Satoshi Nakamoto statue on the city’s beautiful lakefront. Bitcoin’s factions may be at war but there’s no doubt they still worship a common god.
Jeff John Roberts
jeff.roberts@fortune.com
@jeffjohnrobertsDECENTRALIZED NEWS
If you can’t beat ‘em, join ‘em: JPMorgan Chase’s CEO continues to soften his longtime anti-crypto stance as his bank announced that it will let borrowers use Bitcoin and Ethereum for loan collateral by the end of year. (Bloomberg)
COIN upgrade: Coinbase’s forthcoming crypto token could be worth $12 billion to $34 billion, said a JPM analyst, who cited the token and the slowing growth of DEXes as reasons to upgrade the stock ahead of third-quarter earnings this week. (DL News)
Here we ICO again? In assessing Coinbase’s $375 million acquisition of Echo, which was founded by crypto influencer Cobie and helps token projects raise funds, one journalist speculated it could inaugurate the return of 2016-style initial coin offerings. (Bloomberg)
DAT doesn’t add up: Following a Fortune exposé pointing to potential insider trading ahead of public company pivots to digital asset treasuries, a new report provides evidence that insiders tied to some popular DATs are using share sales to circumvent token lockups. (Unchained)
Trump picks a CFTC chair: The White House selected longtime lawyer and crypto guy Mike Selig to lead the agency. The choice of Selig, which came after the Winklevii helped torpedo the original frontrunner, was hailed by industry vets who are eager to finalize a key bill that will divide responsibilities between the SEC and CFTC. (Politico)
MAIN CHARACTER OF THE WEEK
Changpeng Zhao, cofounder of Binance. Samsul Said—Bloomberg/Getty Images
CZ was the easy choice for main character of the week after finally securing a Presidential pardon. Critics, pointing to a $2 billion deal involving the Trump family’s stablecoin and Binance, blasted the pardon as massively corrupt while many on Crypto Twitter claimed it was fair since CZ—who pleaded guilty—had allegedly been the target of a political prosecution.
MEME O’ THE MOMENT

In Lugano, Switzerland, Bitcoiners unveiled a refurbished statue of Satoshi Nakamoto. @Globalstats11
Bitcoin devotees seeking to make a pilgrimage have a growing number of options. In addition to the refurbished Satoshi statue unveiled in Lugano, there is one in Budapest as well. Can a formal shrine—or perhaps a Bitcoin theme park—be far behind?
Continue Reading
-

Mitigation of alkaline stress and iron deficiency in Petunia hybrida through resveratrol-induced physiological and nutrient responses | BMC Plant Biology
Chatterjee S. Petunia. Commercial flowers, vol. 4. New Delhi: Daya Publishing House, A Division of Astral International Pvt. Ltd.; 2022. p. 55.
Guo G, Xiao J, Jeong BR. Iron source and medium pH affect nutrient uptake and pigment content in Petunia hybrida ‘madness red’ cultured in vitro. Int J Mol Sci. 2022;23:8943. https://doi.org/10.3390/ijms23168943.
Google Scholar
Velez Bermudez IC, Schmidt W. Iron sensing in plant. Front Plant Sci. 2023;14:1145510. https://doi.org/10.3389/fpls.2023.1145510.
Google Scholar
Ansari A, Amiri J, Norouzi P, Fattahi M, Easouli-Sadaghiani MH, Alipour H. Assessing the efficacy of different nano-iron sources for alleviating alkaline soil challenges in Goji berry trees (Lycium barbarum L). BMC Plant Biol. 2024;24:1153. https://doi.org/10.1186/s12870-024-05870-3.
Google Scholar
Yang S, Xu Y, Tang Z, Jin S, Yang S. The impact of alkaline stress on plant growth and its alkaline resistance mechanisms. Int J Mol Sci. 2024;25(24):13719. https://doi.org/10.3390/ijms252413719.
Google Scholar
Savchenko T, Tikhonov K. Oxidative stress-induced alteration of plant central metabolism. Life. 2021;11:304. https://doi.org/10.3390/life11040304.
Google Scholar
Bontpart T, Weiss A, Vile D, Gérard F, Lacombe B, Reichheld JP, et al. Growing on calcareous soils and facing climate change. Trends Plant Sci. 2024;29(12):1319–30. https://doi.org/10.1016/j.tplants.2024.03.013.
Google Scholar
Tamir G, Zilkah S, Dai N, Shawahna R, Cohen S, Bar-Tal A. Combined effects of CaCO3 and the proportion of N-NH4+ among the total applied inorganic N on the growth and mineral uptake of rabbiteye blueberry. J Soil Sci Plant Nutr. 2021;21:35–48. https://doi.org/10.1007/s42729-020-00339-2.
Google Scholar
Kumar K, Jaiswal A, Koppolu UMK, Kumar KRR. Alkaline stress disrupts growth, biochemistry, and ion homeostasis of Chickpea (Cicer arietinum L.) roots. Front Agron. 2024;6:1497054. https://doi.org/10.3389/fagro.2024.1497054.
Google Scholar
Zhao Y, Chen Y, Liu S, Li F, Sun M, Liang Z, et al. Bicarbonate rather than high pH in growth medium induced Fe-deficiency chlorosis in dwarfing rootstock quince A (Cydonia oblonga Mill.) but did not impair Fe nutrition of vigorous rootstock Pyrus betulifolia. Front Plant Sci. 2023;14:1237327. https://doi.org/10.3389/fpls.2023.1237327.
Google Scholar
Saleem A, Zulfiqar A, Saleem MZ, Ali B, Saleem MH, Ali S, et al. Alkaline and acidic soil constraints on iron accumulation by rice cultivars in relation to several physio-biochemical parameters. BMC Plant Biol. 2023;23(1):397. https://doi.org/10.1186/s12870-023-04400-x.
Google Scholar
Liang G. Iron uptake, signaling, and sensing in plants. Plant Commun. 2022;3(5):100349. https://doi.org/10.1016/j.xplc.2022.100349.
Google Scholar
Ning X, Lin M, Huang G, Mao J, Gao Z, Wang X. Research progress on iron absorption, transport, and molecular regulation strategy in plants. Front Plant Sci. 2023;14:1190768. https://doi.org/10.3389/fpls.2023.1190768.
Google Scholar
Li J, Cao X, Jia X, Liu L, Cao H, Qin W, et al. Iron deficiency leads to chlorosis through impacting chlorophyll synthesis and nitrogen metabolism in Areca catechu L. Front Plant Sci. 2021a;12:710093. https://doi.org/10.3389/fpls.2021.710093.
Google Scholar
Trofimov K, Mankotia S, Ngigi M, Baby D, Satbhai SB, Bauer P. Shedding light on iron nutrition: exploring intersections of transcription factor cascades in light and iron deficiency signaling. J Exp Bot. 2025;76:787–802. https://doi.org/10.1093/jxb/erae324.
Google Scholar
Khalil S, Strah R, Lodovici A, Vojta P, Ziegler J, Novak MP, Zanin L, Tomasi N, Forneck A, Griesser M. Lime-induced iron deficiency stimulates a stronger response in tolerant grapevine rootstocks compared to low iron availability. Plant Stress. 2025;16:100841. https://doi.org/10.1016/j.stress.2025.100841.
Google Scholar
Martín-Barranco A, Thomine S, Vert G, Zelazny E. A quick journey into the diversity of iron uptake strategies in photosynthetic organisms. Plant Signal Behav. 2021;16(11):1975088. https://doi.org/10.1080/15592324.2021.1975088.
Google Scholar
Amooaghaie R, Roohollahi S. Effect of sodium Nitroprusside on responses of Melissa officinalis to bicarbonate exposure and direct Fe deficiency stress. Photosynthetica. 2017;55(1):153–63. https://doi.org/10.1007/s11099-016-0240-8.
Google Scholar
Wang N, Dong X, Chen Y, Ma B, Yao C, Ma F, et al. Direct and bicarbonate-induced iron deficiency differently affect iron translocation in Kiwifruit roots. Plants. 2020;9:1578. https://doi.org/10.3390/plants9111578.
Google Scholar
Marschner H, Römheld V. Strategies of plants for acquisition of iron. Plant Soil. 1994;165:375–88. https://doi.org/10.1007/BF00008069.
Google Scholar
Kobayashi T, Nakanishi H, Nishizawa NK. Recent insights into iron homeostasis and their application in graminaceous crops. Proc Jpn Acad Ser B. 2010;86:900–13. https://doi.org/10.2183/pjab.86.900.
Google Scholar
Nozoye T, Nagasaka S, Kobayashi T, Takahashi M, Sato Y, Sato Y, et al. Phytosiderophore efflux transporters are crucial for iron acquisition in graminaceous plants. J Biol Chem. 2011;286:5446–54. https://doi.org/10.1074/jbc.M110.180026.
Google Scholar
Wagner ALS, Araniti F, Ishii-Iwamoto EL, Abenavoli MR. Resveratrol exerts beneficial effects on the growth and metabolism of Lactuca sativa L. Plant Physiol Biochem. 2022;171:26–37. https://doi.org/10.1016/j.plaphy.2021.12.023.
Google Scholar
Rao MJ, Zheng B. The role of polyphenols in abiotic stress tolerance and their antioxidant properties to scavenge reactive oxygen species and free radicals. Antioxidants. 2025;14(1):74. https://doi.org/10.3390/antiox14010074.
Google Scholar
Zheng X, Chen H, Su Q, Wang C, Sha G, Ma C, et al. Resveratrol improves the irondeficiency adaptation of Malus baccata seedlings by regulating iron absorption. BMC Plant Biol. 2021;21(1):433. https://doi.org/10.1186/s12870-021-03215-y.
Google Scholar
Šamec D, Karalija E, Šola I, Vujčić Bok V, Salopek-Sondi B. The role of polyphenols in abiotic stress response: the influence of molecular structure. Plants. 2021;10(1):118. https://doi.org/10.3390/plants10010118.
Google Scholar
Jian J, Su W, Liu Y, Wang M, Chen X, Wang E, et al. Effects of saline–alkali composite stress on the growth and soil fixation capacity of four herbaceous plants. Agronomy. 2024;14(7):1556. https://doi.org/10.3390/agronomy14071556.
Google Scholar
López-Pérez M, Acosta J, Pérez-Labrada F. Iron nutrition management in calcisol soils as a tool to mitigate chlorosis and promote crop quality – An overview. J Appl Biol Biotechnol. 2023;12(1):17–29. https://doi.org/10.7324/JABB.2024.157536.
Google Scholar
Mehrotra R, Rajesh KV, Anirban P. Iron deficiency chlorosis in aromatic grasses—A review. Environ Chall. 2022;9:100646. https://doi.org/10.1016/j.envc.2022.100646.
Google Scholar
Liu X, Niu H, Li J, Jiang D, Chen R, Zhang R, et al. Higher endogenous abscisic acid confers greater tolerance to saline-alkaline stress in Petunia hybrida. Environ Exp Bot. 2024;228:106035. https://doi.org/10.1016/j.envexpbot.2024.106035.
Google Scholar
Murata Y, Itoh Y, Iwashita T, Namba K. Transgenic petunia with the iron(III)phytosiderophore transporter gene acquires tolerance to iron deficiency in alkaline environments. PLoS ONE. 2015;10:e0120227. https://doi.org/10.1371/journal.pone.0120227.
Google Scholar
Jelali N, Wasli H, Youssef RB, Hessini K, Cardoso SM. Iron deficiency modulates secondary metabolite biosynthesis and antioxidant potential in Sulla carnosa L. primed with Salicylic acid. Appl Sci. 2022;12(20):10351. https://doi.org/10.3390/app122010351.
Google Scholar
Sun Z, Wang T, Li J, Zheng X, Ge H, Sha G, et al. Resveratrol enhances the tolerance of Malus hupehensis to potassium deficiency stress. Front Plant Sci. 2024;15:1503463. https://doi.org/10.3389/fpls.2024.1503463.
Google Scholar
Li T, Li Y, Sun Z, Xi X, Sha G, Ma C, et al. Resveratrol alleviates the KCl salinity stress of Malus hupehensis Rhed. Front Plant Sci. 2021b;12:650485. https://doi.org/10.3389/fpls.2021.650485.
Google Scholar
Hoagland DR, Arnon DI. The waterculture method for growing plants without soil. Berkeley (CA): California Agricultural Experiment Station; 1950. Circular No. 347. 32.
Sonneveld C, Straver N. Nutrient solutions for vegetables and flowers grown in water or substrates. Naaldwijk (Netherlands): Glasshouse Crops Research Station; 1999. p. 43.
Poorter H, Niinemets Ü, Poorter L, Wright IJ, Villar R. Causes and consequences of variation in leaf mass per area (LMA): a metaanalysis. New Phytol. 2009;182(3):565–88. https://doi.org/10.1111/j.1469-8137.2009.02830.x.
Google Scholar
Pang W, Crow WT, Luc JE, McSorley R, GiblinDavis RM, Kenworthy KE, et al. Comparison of water displacement and WinRHIZO software for plant root parameter assessment. Plant Dis. 2011;95(10):1308–10. https://doi.org/10.1094/PDIS-01-11-0026.
Google Scholar
Markwell J, Osterman JC, Mitchell JL. Calibration of the Minolta SPAD-502 leaf chlorophyll meter. Photosynth Res. 1995;46:467–72. https://doi.org/10.1007/BF00032301.
Google Scholar
Lichtenthaler HK. Chlorophylls and carotenoids: pigments of photosynthetic biomembranes. Methods Enzymol. 1987;148:350–82. https://doi.org/10.1016/0076-6879(87)48036-1.
Google Scholar
Lutts S, Kinet JM, Bouharmont J. Changes in plant response to NaCl during development of rice (Oryza sativa L.) varieties differing in salinity resistance. J Exp Bot. 1995;46(12):1843–52. https://doi.org/10.1093/jxb/46.12.1843.
Google Scholar
Horst JH, Cakmak I. Effects of aluminum on lipid peroxidation, superoxide dismutase, catalase, and peroxidase activities in root tips of soybean (Glycine max). Physiol Plant. 1991;83:463–8. https://doi.org/10.1111/j.1399-3054.1991.tb00121.x.
Google Scholar
Velikova V, Yordanov I, Edreva A. Oxidative stress and some antioxidant systems in acid rain-treated bean plants: protective role of exogenous polyamines. Plant Sci. 2000;151(1):59–66. https://doi.org/10.1016/S0168-9452(99)00197-1.
Google Scholar
Ojeda M, Schaffer B, Davies FS. Root and leaf ferric chelate reductase activity in pond Apple and soursop. J Plant Nutr. 2004;27:1381–93. https://doi.org/10.1081/PLN-200025836.
Google Scholar
Grieve CM, Grattan SR. Rapid assay for determination of water-soluble quaternary ammonium compounds. Plant Soil. 1983;70(3):303–7. https://doi.org/10.1007/BF02374789.
Google Scholar
Ohayama T, Ito M, Kobayashi K, Araki S, Yasuyoshi S, Sasaki O, et al. Analytical procedures of N, P and K content in plant and manure materials using H₂SO₄–H₂O₂ Kjeldahl digestion method. Bull Fac Agric Niigata Univ. 1991;43:111–20.
Ryan J, Estefan G, Rashid A. Soil and plant analysis: laboratory manual. Aleppo (Syria): ICARDA; 2001.
Mizukoshi K, Nishiwaki T, Ohtake N, Minagawa R, Kobayashi K, Ikarashi T, et al. Determination of tungstate concentration in plant materials by HNO₃–HClO₄ digestion and colorimetric method using thiocyanate. Plant Anal Methods. 1994;46:51–6.
Ghazanshahi J. Soil and plant analysis. Tehran (Iran): Motarjem; 2006. p. 311.
Ahmed N, Zhang B, Chachar Z, Li J, Xiao G, Wang Q, et al. Micronutrients and their effects on horticultural crop quality, productivity and sustainability. Sci Hortic. 2024;323:112512. https://doi.org/10.1016/j.scienta.2023.112512.
Google Scholar
Khan F, Siddique AB, Shabala S, Zhou M, Zhao C. Phosphorus plays key roles in regulating plants’ physiological responses to abiotic stresses. Plants. 2023;12(15):2861. https://doi.org/10.3390/plants12152861.
Google Scholar
Therby-Vale R, Lacombe B, Rhee SY, Nussaume L, Rouached H. Mineral nutrient signaling controls photosynthesis: focus on iron deficiency-induced chlorosis. Trends Plant Sci. 2022;27(5):502–9. https://doi.org/10.1016/j.tplants.2021.11.005.
Google Scholar
Hasanuzzaman M, Bhuyan MHMB, Parvin K, Bhuiyan TF, Anee TI, Nahar K, et al. Regulation of ROS metabolism in plants under environmental stress: a review of recent experimental evidence. Int J Mol Sci. 2020a;21(22):8695. https://doi.org/10.3390/ijms21228695.
Google Scholar
Hong Y, Boiti A, Vallone D, Foulkes NS. Reactive oxygen species signaling and oxidative stress: transcriptional regulation and evolution. Antioxidants. 2024;13(3):312. https://doi.org/10.3390/antiox13030312.
Google Scholar
Saito A, Shinjo S, Ito D, Doi Y, Sato A, Wakabayashi Y, et al. Enhancement of photosynthetic iron-use efficiency is an important trait of Hordeum vulgare for adaptation of photosystems to iron deficiency. Plants. 2021;10(2):234. https://doi.org/10.3390/plants10020234.
Google Scholar
Marschner P. Marschner’s mineral nutrition of higher plants. 3rd ed. San Diego: Academic; 2012. https://doi.org/10.1016/C2009-0-63043-9.
Google Scholar
Zheng L, Yamaji N, Ma JF. Iron transport and distribution in plants: research progress and future perspectives. Plant Cell Physiol. 2022;63(2):185–93. https://doi.org/10.1093/pcp/pcab164.
Google Scholar
Giehl RF, Lima JE, von Wirén N. Localized iron supply triggers lateral root elongation in Arabidopsis by altering the AUX1-mediated auxin distribution. Plant Cell. 2012;24(1):33–49. https://doi.org/10.1105/tpc.111.092973.
Google Scholar
Yang C, Shi D, Wang D. Comparative effects of salt and alkali stresses on growth, osmotic adjustment and ionic balance of an alkali-resistant halophyte Suaeda glauca (Bge). Plant Growth Regul. 2008;56:179–90. https://doi.org/10.1007/s10725-008-9299-y.
Google Scholar
Sun X, Zhu C, Li B, Ning W, Yin J. Combining physiology and transcriptome to reveal mechanisms of Hosta ‘golden cadet’ in response to alkali stress. Plants. 2025;14(4):593. https://doi.org/10.3390/plants14040593.
Google Scholar
Yang Y, Ian J, Qiu X, Wang G, Zong J. Effects of combined saline-alkali stress on physiological and biochemical characteristics of OT hybrid Lily. J Nanjing Univ. 2022;46(4):117. https://doi.org/10.12302/j.issn.1000-2006.202105041.
Google Scholar
Gao Q, Zheng R, Lu J, Li X, Wang D, Cai X, et al. Trends in the potential of stilbenes to improve plant stress tolerance: insights of plant defense mechanisms in response to biotic and abiotic stressors. J Agric Food Chem. 2024;72(14):7655–71. https://doi.org/10.1021/acs.jafc.4c00326.
Google Scholar
Vélez-Bermúdez IC, Schmidt W. Plant strategies to mine iron from alkaline substrates. Plant Soil. 2023;483:1–25. https://doi.org/10.1007/s11104-022-05746-1.
Google Scholar
Rottet S, Förster B, Hee WY, Rourke LM, Price GD, Long BM. Engineered accumulation of bicarbonate in plant chloroplasts: known knowns and known unknowns. Front Plant Sci. 2021;12:727118. https://doi.org/10.3389/fpls.2021.727118.
Google Scholar
Bhat MA, Mishra AK, Shah SN, Bhat MA, Jan S, Rahman S, et al. Soil and mineral nutrients in plant health: a prospective study of iron and phosphorus in the growth and development of plants. Curr Issues Mol Biol. 2024;46(6):5194–222. https://doi.org/10.3390/cimb46060312.
Google Scholar
Rengasamy P, Lacerda C, Gheyi H. Salinity, sodicity and alkalinity. Subsoil constraints for crop production. Cham: Springer; 2022. pp. 75–94. https://doi.org/10.1007/978-3-031-00317-2_4.
Google Scholar
Zagoskina NV, Zubova MY, Nechaeva TL, Kazantseva VV, Goncharuk EA, Katanskaya VM, et al. Polyphenols in plants: structure, biosynthesis, abiotic stress regulation, and practical applications. Int J Mol Sci. 2023;24(18):13874. https://doi.org/10.3390/ijms241813874.
Google Scholar
Chauhan J, Prathibha MD, Singh P, Choyal P, Mishra UN, Saha D, et al. Plant photosynthesis under abiotic stresses: damages, adaptive, and signaling mechanisms. Plant Stress. 2023;10:100296. https://doi.org/10.1016/j.stress.2023.100296.
Google Scholar
Graziano M, Lamattina L. Nitric oxide and iron in plants: an emerging and converging story. Trends Plant Sci. 2005;10:4–8. https://doi.org/10.1016/j.tplants.2004.12.004.
Google Scholar
Tripathy BC, Oelmüller R. Reactive oxygen species generation and signaling in plants. Plant Signal Behav. 2012;7(12):1621–33. https://doi.org/10.4161/psb.22455.
Google Scholar
Apel K, Hirt H. Reactive oxygen species: metabolism, oxidative stress, and signal transduction. Annu Rev Plant Biol. 2004;55:373–99. https://doi.org/10.1146/annurev.arplant.55.031903.141701.
Google Scholar
Ahuja I, Kissen R, Bones AM. Phytoalexins in defense against pathogens. Trends Plant Sci. 2012;17(2):73–90. https://doi.org/10.1016/j.tplants.2011.11.002.
Google Scholar
Jeandet P, Douillet-Breuil AC, Bessis R, Debord S, Sbaghi M, Adrian M. Phytoalexins from the vitaceae: biosynthesis, phytoalexin gene expression in Transgenic plants, antifungal activity, and metabolism. J Agric Food Chem. 2013;51(20):6109–15. https://doi.org/10.1021/jf011429s.
Google Scholar
Kong Q, Zheng S, Li W, Liang H, Zhou L, Yang H, et al. Performance of Camellia oleifera seedlings under alkali stress improved by spraying with types of exogenous biostimulants. Agriculture. 2025;15(3):274. https://doi.org/10.3390/agriculture15030274.
Google Scholar
Arcas A, López-Rayo S, Gárate A, Lucena JJ. A critical review of methodologies for evaluating iron fertilizers based on iron reduction and uptake by strategy i plants. Plants. 2024;13(6):819. https://doi.org/10.3390/plants13060819.
Google Scholar
Kobayashi T, Nishizawa NK. Iron uptake, translocation, and regulation in higher plants. Annu Rev Plant Biol. 2012;63:131–52. https://doi.org/10.1146/annurev-arplant-042811-105522.
Google Scholar
Santi S, Schmidt W. Dissecting iron deficiency-induced proton extrusion in Arabidopsis roots. New Phytol. 2009;183(4):1072–84. https://doi.org/10.1111/j.1469-8137.2009.02901.x.
Google Scholar
Hsieh EJ, Waters BM. Alkaline stress and iron deficiency regulate iron uptake and riboflavin synthesis gene expression differently in root and leaf tissue: implications for iron deficiency chlorosis. J Exp Bot. 2016;67(19):5671–85. https://doi.org/10.1093/jxb/erw328.
Google Scholar
Ashraf M, Foolad MR. Roles of glycine betaine and proline in improving plant abiotic stress resistance. Environ Exp Bot. 2007;59(2):206–16. https://doi.org/10.1016/j.envexpbot.2005.12.006.
Google Scholar
Zhu XG, Long SP, Ort DR. Improving photosynthetic efficiency for greater yield. Annu Rev Plant Biol. 2016;61:235–61. https://doi.org/10.1146/annurev-arplant-042809-112206.
Google Scholar
Truong VL, Jun M, Jeong WS. Role of resveratrol in regulation of cellular defense systems against oxidative stress. Biofactors. 2018;44(1):36–49. https://doi.org/10.1002/biof.1399.
Google Scholar
D’Introno A, Paradiso A, Scoditti E, D’Amico L, De Paolis A, Carluccio MA, et al. Antioxidant and anti-inflammatory properties of tomato fruits synthesizing different amounts of Stilbenes. Plant Biotechnol J. 2009;7(5):422–9. https://doi.org/10.1111/j.1467-7652.2009.00409.x.
Google Scholar
Shi Y, Guo S, Zhao X, Xu M, Xu J, Xing G, Ahammed GJ. Comparative physiological and transcriptomics analysis revealed crucial mechanisms of silicon-mediated tolerance to iron deficiency in tomato. Front Plant Sci. 2022;13:1094451. https://doi.org/10.3389/fpls.2022.1094451.
Google Scholar
Johan PD, Ahmed OH, Omar L, Hasbullah NA. Phosphorus transformation in soils following co-application of charcoal and wood ash. Agronomy. 2021;11(10):2010. https://doi.org/10.3390/agronomy11102010.
Google Scholar
Santoro V, Schiavon M, Celi L. Role of soil abiotic processes on phosphorus availability and plant responses with a focus on Strigolactones in tomato plants. Plant Soil. 2024;494:1–49. https://doi.org/10.1007/s11104-023-06266-2.
Google Scholar
Zhao H, Zhang W, Zhang L. Interactive effects of iron deficiency and other mineral nutrients on plants. Plant Soil. 2014;382(1–2):1–19. https://doi.org/10.1007/s11104-014-2152-1.
Google Scholar
Wdowiak A, Podgórska A, Szal B. Calcium in plants: an important element of cell physiology and structure, signaling, and stress responses. Acta Physiol Plant. 2024;46:108. https://doi.org/10.1007/s11738-024-03733-w.
Google Scholar
Zhang X, Zhang D, Sun W, Wang T. The adaptive mechanism of plants to iron deficiency via iron uptake, transport, and homeostasis. Int J Mol Sci. 2019;20(10):2424. https://doi.org/10.3390/ijms20102424.
Google Scholar
Ahmed N, Zhang B, Bozdar B, Chachar S, Rai M, Li J, et al. The power of magnesium: unlocking the potential for increased yield, quality, and stress tolerance of horticultural crops. Front Plant Sci. 2023;14:1285512. https://doi.org/10.3389/fpls.2023.1285512.
Google Scholar
Cakmak I. Enrichment of cereal grains with zinc: agronomic or genetic biofortification? Plant Soil. 2008;302(1–2):1–17. https://doi.org/10.1007/s11104-007-9466-3.
Google Scholar
Rai S, Singh PK, Mankotia S, Swain J, Satbhai SB. Iron homeostasis in plants and its crosstalk with copper, zinc, and manganese. Plant Stress. 2021;1:100008. https://doi.org/10.1016/j.stress.2021.100008.
Google Scholar
Shaver TM, Westfall D, Ronaghi M. Zinc fertilizer solubility and its effects on zinc bioavailability over time. J Plant Nutr. 2007;30:123–33. https://doi.org/10.1080/01904160601055145.
Google Scholar
Garcia-Caparros P, Ciriello M, Rouphael Y, Giordano M. The role of organic extracts and inorganic compounds as alleviators of drought stress in plants. Horticulturae. 2025;11(1):91. https://doi.org/10.3390/horticulturae11010091.
Google Scholar
Jeandet P. Phytoalexins. Current progress and future prospects. Mol. 2015;20(2):2770–4. https://doi.org/10.3390/molecules20022770.
Google Scholar
Chang X, Heene E, Qiao F, Nick P. The phytoalexin Resveratrol regulates the initiation of hypersensitive cell death in Vitis cell. PLoS ONE. 2011;6(10):e26405. https://doi.org/10.1371/journal.pone.0026405.
Google Scholar
Stanton C, Sanders D, Kraemer U, Podar D. Zinc in plants: integrating homeostasis and biofortification. Mol Plant. 2022;15(1):65–85. https://doi.org/10.1016/j.molp.2021.12.008.
Google Scholar
Xu L, Wang X. A comprehensive review of phenolic compounds in horticultural plants. Int J Mol Sci. 2025;26:5767. https://doi.org/10.3390/ijms26125767.
Google Scholar
Continue Reading
-
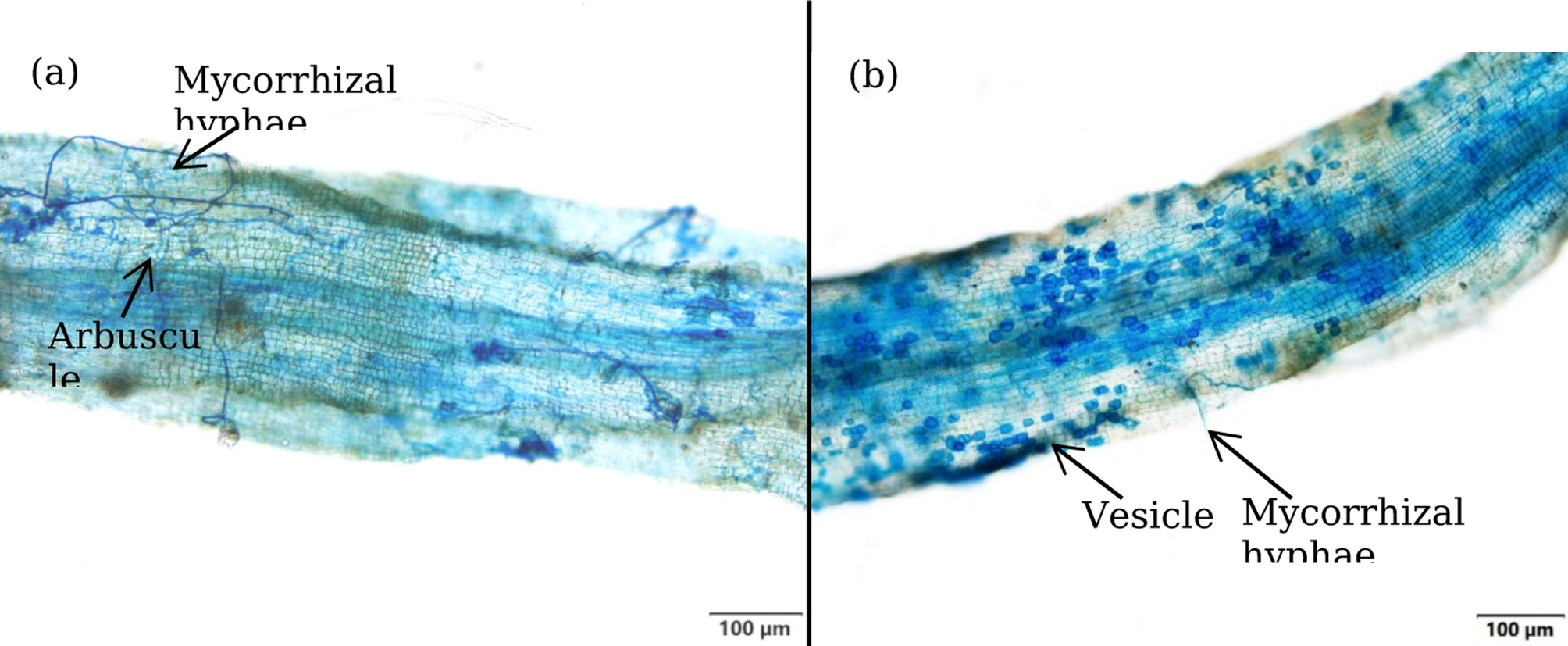
Arbuscular mycorrhizal fungi mediate leaf sugar profile in water-stressed trifoliate orange | BMC Plant Biology
Soka G, Ritchie M. Arbuscular mycorrhizal symbiosis, ecosystem processes and environmental changes in tropical soils. Appl Ecol Environ Res. 2015;13:229–45.
Begum N, Qin C, Ahanger MA, Raza S,…
Continue Reading
-

Why Don’t Atmospheric Greenhouse Gases Saturate, As Some Predicted?
Anytime global warming or climate change get mentioned online, you can expect a bunch of comments claiming to be able to disprove the whole concept, at least in relation to human activity. Most of these have been debunked, not just in detailed…
Continue Reading