This request seems a bit unusual, so we need to confirm that you’re human. Please press and hold the button until it turns completely green. Thank you for your cooperation!
Blog
-
Just a moment…
Just a moment… -

Pat Cummins concedes he is ‘weeks away’ from bowling and unlikely to play in first Ashes Test | Australia cricket team
Pat Cummins has admitted he is unlikely to play in the Ashes opener, conceding a return to proper bowling is still some time away.
Cummins is expected to learn this week whether he will be able to feature in Perth on 21 November, with officials…
Continue Reading
-

Quote & Analysis: Magic’s Unselfishness, Franz’s Free Throw Rate, Jase’s 3-Point Shooting & More – NBA
- Quote & Analysis: Magic’s Unselfishness, Franz’s Free Throw Rate, Jase’s 3-Point Shooting & More NBA
- Orlando Magic continue putting biggest weakness to rest Orlando Magic Daily
- 2025-26 NBA Guide- Orlando Magic The Playoffs
- Orlando Magic…
Continue Reading
-
Live updates: Trump declares ‘dawn of a new Middle East,’ celebrating release of all living hostages – The Washington Post
- Live updates: Trump declares ‘dawn of a new Middle East,’ celebrating release of all living hostages The Washington Post
- LIVE: After Israel, Trump arrives in Egypt for Gaza ceasefire summit Al Jazeera
- Next 24 hours: Three things to watch on…
Continue Reading
-
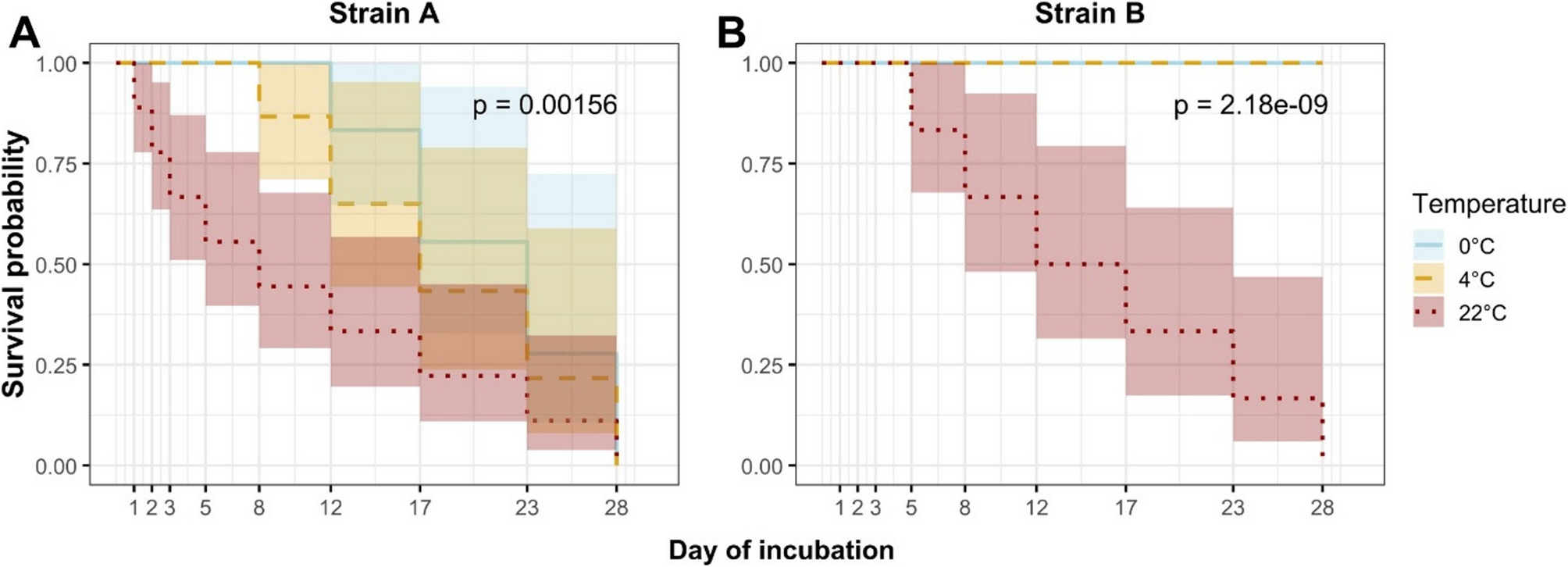
Temperature-dependent survival of Mycoplasma anserisalpingitidis in water: implications for biosecurity and transmission in waterfowl farming | BMC Veterinary Research
Ferguson-Noel N, Armour NK, Noormohammadi AH, El-Gazzar M, Bradbury JM. Mycoplasmosis. In: Swayne DE, editor. Diseases of Poultry. 14th ed. Hoboken, NJ, USA: John Wiley & Sons, Inc.; 2020. p. 907–65.
Volokhov DV, Grózner D, Gyuranecz M, Ferguson-Noel N, Gao Y, Bradbury JM, et al. Mycoplasma anserisalpingitidis sp. nov., isolated from European domestic geese (Anser anser domesticus) with reproductive pathology. Int J Syst Evol Microbiol. 2020;70:2369–81. https://doi.org/10.1099/ijsem.0.004052.
Google Scholar
Sawicka-Durkalec A, Tomczyk G, Kursa O, Stenzel T, Gyuranecz M. Evidence of Mycoplasma spp. Transmission by migratory wild geese. Poult Sci. 2022;101:101526. https://doi.org/10.1016/j.psj.2021.101526.
Google Scholar
Polak-Vogelzang AA. Survival of Mycoplasma gallisepticum in mains water. Avian Pathol. 1977;6:93–5. https://doi.org/10.1080/03079457708418215.
Google Scholar
Marois C, Savoye C, Kobisch M, Kempf I. A reverse transcription-PCR assay to detect viable Mycoplasma synoviae in poultry environmental samples. Vet Microbiol. 2002;89:17–28. https://doi.org/10.1016/S0378-1135(02)00159-1.
Google Scholar
Marois C, Dufour-Gesbert F, Kempf I. Polymerase chain reaction for detection of Mycoplasma gallisepticum in environmental samples. Avian Pathol. 2002;31:163–8. https://doi.org/10.1080/03079450120118658.
Google Scholar
Marois C, Picault J-P, Kobisch M, Kempf I. Experimental evidence of indirect transmission of Mycoplasma synoviae. Vet Res. 2005;36:759–69. https://doi.org/10.1051/vetres:2005031.
Google Scholar
Münster P, Kemper N. Long-term analysis of drinking water quality in poultry and pig farms in Northwest Germany. Front Anim Sci. 2024;5:1467287. https://doi.org/10.3389/fanim.2024.1467287.
Google Scholar
Elmberg J, Berg C, Lerner H, Waldenström J, Hessel R. Potential disease transmission from wild geese and swans to livestock, poultry and humans : a review of the scientific literature from a one health perspective. Infect Ecol Epidemiol. 2017;7. https://doi.org/10.1080/20008686.2017.1300450.
Abulreesh HH, Paget TA, Goulder R. Waterfowl and the bacteriological quality of amenity ponds. J Water Health. 2004;2:183–9. https://doi.org/10.2166/wh.2004.0016.
Google Scholar
Gonzalez JM, Aranda B. Microbial growth under limiting Conditions-Future perspectives. Microorganisms. 2023;11:1641. https://doi.org/10.3390/microorganisms11071641.
Google Scholar
Arana I, Muela A, Orruño M, Seco C, Garaizabal I, Barcina I. Effect of temperature and starvation upon survival strategies of Pseudomonas fluorescens CHA0: comparison with Escherichia coli. FEMS Microbiol Ecol. 2010;74:500–9. https://doi.org/10.1111/j.1574-6941.2010.00979.x.
Google Scholar
Nedwell DB. Effect of low temperature on microbial growth: Lowered affinity for substrates limits growth at low temperature. FEMS Microbiol Ecol. 2006;30:101–11. https://doi.org/10.1111/j.1574-6941.1999.tb00639.x.
Google Scholar
Marmion M, Macori G, Ferone M, Whyte P, Scannell AGM. Survive and thrive: control mechanisms that facilitate bacterial adaptation to survive manufacturing-related stress. Int J Food Microbiol. 2022;368:109612. https://doi.org/10.1016/j.ijfoodmicro.2022.109612.
Google Scholar
Moon S, Ham S, Jeong J, Ku H, Kim H, Lee C. Temperature matters: bacterial response to temperature change. J Microbiol. 2023;61:343–57. https://doi.org/10.1007/s12275-023-00031-x.
Google Scholar
Citti C, Blanchard A. Mycoplasmas and their host: emerging and re-emerging minimal pathogens. Trends Microbiol. 2013;21:196–203. https://doi.org/10.1016/j.tim.2013.01.003.
Google Scholar
Rocha EPC, Blanchard A. Genomic repeats, genome plasticity and the dynamics of Mycoplasma evolution. Nucleic Acids Res. 2002;30:2031–42. https://doi.org/10.1093/nar/30.9.2031.
Google Scholar
Katz SD. The Streak Plate Protocol. Am Soc Microbiol. 2008;1–10. https://asm.org/asm/media/protocol-images/the-streak-plate-protocol.pdf.
Grózner D, Sulyok KM, Kreizinger Z, Rónai Z, Jánosi S, Turcsányi I, et al. Detection of Mycoplasma anatis, M. anseris, M. cloacale and Mycoplasma sp. 1220 in waterfowl using species-specific PCR assays. PLoS ONE. 2019;14:e0219071. https://doi.org/10.1371/journal.pone.0219071.
Google Scholar
Gioia G, Werner B, Nydam DV, Moroni P. Validation of a Mycoplasma molecular diagnostic test and distribution of Mycoplasma species in bovine milk among new York state dairy farms. J Dairy Sci. 2016;99:4668–77. https://doi.org/10.3168/jds.2015-10724.
Google Scholar
Hannan PCT. Guidelines and recommendations for antimicrobial minimum inhibitory concentration (MIC) testing against veterinary Mycoplasma species. Vet Res. 2000;31:373–95. https://doi.org/10.1051/vetres:2000100.
Google Scholar
Bekő K, Grózner D, Mitter A, Udvari L, Földi D, Wehmann E, et al. Development and evaluation of temperature-sensitive Mycoplasma anserisalpingitidis clones as vaccine candidates. Avian Pathol. 2022;51:535–49. https://doi.org/10.1080/03079457.2022.2102967.
Google Scholar
Terry M. Therneau, Patricia M. Grambsch. Modeling Survival Data: Extending the Cox Model. New York: Springer; 2000.
Tang Y, Horikoshi M, Li W. Ggfortify: unified interface to visualize statistical results of popular R packages. R J. 2016;8:474. https://doi.org/10.32614/RJ-2016-060.
Google Scholar
Wickham H. ggplot2: Elegant Graphics for Data Analysis. New York: Springer-Verlag; 2016. https://doi.org/10.1007/978-3-319-24277-4.
R Core Team. R: A Language and environment for statistical computing. Vienna, Austria: Foundation for Statistical Computing; 2025.
Posit team. RStudio: Integrated Development Environment for R. Posit Software, PBC, Boston: MA; 2025. http://www.posit.co/.
Merchant SS, Helmann JD. Elemental economy: microbial strategies for optimizing growth in the face of nutrient limitation. Adv Microb Physiol. 2012;91–210. https://doi.org/10.1016/B978-0-12-398264-3.00002-4.
Nagatomo H. Comparative studies of the persistence of animal Mycoplasmas under different environmental conditions. Vet Microbiol. 2001;82:223–32. https://doi.org/10.1016/S0378-1135(01)00385-6.
Google Scholar
Justice-Allen A, Trujillo J, Corbett R, Harding R, Goodell G, Wilson D. Survival and replication of Mycoplasma species in recycled bedding sand and association with mastitis on dairy farms in Utah. J Dairy Sci. 2010;93:192–202. https://doi.org/10.3168/jds.2009-2474.
Google Scholar
Nouvel LX, Sirand-Pugnet P, Marenda MS, Sagné E, Barbe V, Mangenot S, et al. Comparative genomic and proteomic analyses of two Mycoplasma agalactiae strains: clues to the macro- and micro-events that are shaping Mycoplasma diversity. BMC Genomics. 2010;11:86. https://doi.org/10.1186/1471-2164-11-86.
Google Scholar
Delaney NF, Balenger S, Bonneaud C, Marx CJ, Hill GE, Ferguson-Noel N, et al. Ultrafast evolution and loss of crisprs following a host shift in a novel wildlife pathogen, Mycoplasma gallisepticum. PLoS Genet. 2012;8:e1002511. https://doi.org/10.1371/journal.pgen.1002511.
Google Scholar
Bekő K, Nagy EZ, Grózner D, Kreizinger Z, Gyuranecz M. Biofilm formation and its impact on environmental survival and antibiotic resistance of Mycoplasma anserisalpingitidis strains. Acta Vet Hung. 2022;70:184–91. https://doi.org/10.1556/004.2022.00029.
Google Scholar
Rossi C, Chaves-López C, Serio A, Goffredo E, Cenci Goga BT, Paparella A. Influence of incubation conditions on biofilm formation by Pseudomonas fluorescens isolated from dairy products and dairy manufacturing plants. Ital J Food Saf. 2016;5. https://doi.org/10.4081/ijfs.2016.5793.
De Plano LM, Caratozzolo M, Conoci S, Guglielmino SPP, Franco D. Impact of nutrient starvation on biofilm formation in Pseudomonas aeruginosa: an analysis of growth, adhesion, and Spatial distribution. Antibiotics. 2024;13:987. https://doi.org/10.3390/antibiotics13100987.
Google Scholar
Catania S, Bottinelli M, Fincato A, Tondo A, Matucci A, Nai G, et al. Pathogenic avian Mycoplasmas show phenotypic differences in their biofilm forming ability compared to non-pathogenic species in vitro. Biofilm. 2024;7:100190. https://doi.org/10.1016/j.bioflm.2024.100190.
Google Scholar
Pletnev P, Osterman I, Sergiev P, Bogdanov A, Dontsova O. Survival guide: Escherichia coli in the stationary phase. Acta Naturae. 2015;7:22–33.
Google Scholar
Navarro Llorens JM, Tormo A, Martínez-García E. Stationary phase in gram-negative bacteria. FEMS Microbiol Rev. 2010;34:476–95. https://doi.org/10.1111/j.1574-6976.2010.00213.x.
Google Scholar
Hazan R, Schoemann M, Klutstein M. Endurance of extremely prolonged nutrient prevention across kingdoms of life. iScience. 2021;24:102745. https://doi.org/10.1016/j.isci.
Google Scholar
Chernov VM, Gogolev YV, Mukhametshina NE, Abdrakhimov FA, Chernova OA. Mycoplasma adaptation to biogenic and abiogenic stessful factors; Acholeplasma laidlawii nannotransformation and minibodies. Prog Nucl Energy 6 Biol Sci. 2003;396:417–20. https://doi.org/10.0012/4966/04/0506-0251.
Demina IA, Serebryakova MV, Ladygina VG, Rogova MA, Kondratov IG, Renteeva AN, et al. Proteomic characterization of Mycoplasma gallisepticum nanoforming. Biochem (Moscow). 2010;75:1252–7. https://doi.org/10.1134/S0006297910100068.
Google Scholar
Chernov VM, Chernova OA, Gorshkov OV, Muzykantov AA, Shaimardanova GF, Pel’nikevich AD, et al. Adaptation of Mycoplasma gallisepticum to unfavorable growth conditions: changes in morphological and physiological characteristics. Microbiol (N Y). 2008;77:691–4. https://doi.org/10.1134/S0026261708060064.
Google Scholar
Chernov VM, Chernova OA, Medvedeva ES, Sorvina AI, Davydova MN, Rogova MA, et al. Responses of Acholeplasma Laidlawii PG8 cells to cold shock and oxidative stress: proteomic analysis and stress-reactive Mycoplasma proteins. Dokl Biochem Biophys. 2010;432:126–30. https://doi.org/10.1134/S1607672910030099.
Google Scholar
Piccirillo A, Tolosi R, Mughini-Gras L, Kers JG, Laconi A. Drinking water and biofilm as sources of antimicrobial resistance in Free-Range organic broiler farms. Antibiotics. 2024;13:808. https://doi.org/10.3390/antibiotics13090808.
Google Scholar
Mustedanagic A, Matt M, Weyermair K, Schrattenecker A, Kubitza I, Firth CL, et al. Assessment of microbial quality in poultry drinking water on farms in Austria. Front Vet Sci. 2023;10:1254442. https://doi.org/10.3389/fvets.2023.1254442.
Google Scholar
Kapperud G, Skjerve E, Vik L, Hauge K, Lysaker A, Aalmen I, et al. Epidemiological investigation of risk factors for Campylobacter colonization in Norwegian broiler flocks. Epidemiol Infect. 1993;111:245–55. https://doi.org/10.1017/s0950268800056958.
Google Scholar
Sparks NHC. The role of the water supply system in the infection and control of Campylobacter in chicken. Worlds Poult Sci J. 2009;65:459–74. https://doi.org/10.1017/S0043933909000324.
Google Scholar
Gbylik-Sikorska M, Posyniak A, Sniegocki T, Sell B, Gajda A, Sawicka A, et al. Influence of enrofloxacin traces in drinking water to doxycycline tissue pharmacokinetics in healthy and infected by Mycoplasma gallisepticum broiler chickens. Food Chem Toxicol. 2016;90:123–9. https://doi.org/10.1016/j.fct.2016.02.006.
Google Scholar
Continue Reading
-

Sugary and sweetened drinks increase liver disease risk
Are sweetened drinks worse than sugary ones? Key Liver Risk Summary
- Sweetened drinks raise liver disease risk by 60 percent daily intake
- Sugary drinks increase liver disease risk by 50 percent per day
- Sweetened drinks linked to liver-related deaths,…
Continue Reading
-
Association between the inflammatory burden index and erectile dysfunction: a cross-sectional study based on NHANES 2001–2004 | Journal of Health, Population and Nutrition
Wang X, He Z, Liao C, Guo P, Zhao Y, Xiong W. A cross-sectional analysis of the association between screen-based sedentary behavior and erectile dysfunction in US adult males. Sci Rep. 2025;15(1):18060.
…
Continue Reading
-

Boeing Company – Boeing, Airline Partners Set New Standard for Parts Authentication
PLANO, Texas, Oct. 13, 2025 /PRNewswire/ — Boeing [NYSE: BA], in partnership with Southwest Airlines (SWA) and Aeroxchange Ltd., has successfully completed the aerospace industry’s first parts shipment accompanied by a digital 8130-3 certificate—an electronic version of the FAA-governed 8130-3 Authorized Release Certificate. This milestone advances supply chain security by preventing unapproved spare parts from entering the aerospace aftermarket.
“This industry-first shipment reflects Boeing’s dedication to pursuing game-changing solutions through teamwork and partnership,” said William Ampofo, senior vice president, Parts & Distribution and Supply Chain, Boeing Global Services. “Together with Southwest Airlines and Aeroxchange, we are transforming how the industry ensures part authenticity and supply chain security.”
The FAA Form 8130-3 certifies the airworthiness of aircraft parts, components and articles. The digital 8130 certificate replaces the traditional paper certificate with a secure, encrypted file that authenticates the authorized signer’s identity and ensures document integrity. Boeing led a pilot project to generate and gain authorization for this digital solution.
Recently, a battery serviced at Boeing’s product repair services center in Davie, Florida, was the first part shipped using the electronic form, transmitted using the Aeroxchange eARC™ platform. Southwest Airlines received the battery at its Dallas facility, verifying its authenticity and airworthiness through the new digital process.
“Southwest is proud to be a partner in the electronic process of document transfers and thrilled to be onsite for the very first delivery of a ship battery using this process. The security benefit of electronic forms aligns to Southwest’s value of a Safety-first culture and will be of significant benefit in the aviation industry,” said Landon Nitschke, senior vice president, Technical Operations, Southwest Airlines.
“Aeroxchange is honored to have partnered with Boeing and Southwest Airlines to transmit this first ever eARC document providing a highly secure, verifiable digital record of the Authorized Release Certificate, Form 8130-3,” said Al Koszarek, president and CEO of Aeroxchange. “This landmark event is a milestone on the industry’s journey to prevent unapproved parts from entering the aviation supply chain.”
Leveraging industry-leading X.509 security protocols, public/private key encryption, and blockchain-ready formats, the digital 8130 certificate creates an immutable, verifiable record of part authenticity throughout its lifecycle.
Boeing will continue rolling out use of the digital 8130 certificate across all nine of its product repair services centers, as each center receives authorization from the FAA to use electronic systems for recordkeeping, electronic signatures and electronic manuals.
Expanding the use of digital authorized release certificates was a key recommendation from the Aviation Supply Chain Integrity Coalition (ASCIC), a cross-industry group dedicated to preventing unapproved parts from entering the aviation supply chain. Boeing, Southwest Airlines and Aeroxchange are active members of the ASCIC.
A leading global aerospace company and top U.S. exporter, Boeing develops, manufactures and services commercial airplanes, defense products and space systems for customers in more than 150 countries. Our U.S. and global workforce and supplier base drive innovation, economic opportunity, sustainability and community impact. Boeing is committed to fostering a culture based on our core values of safety, quality and integrity.
Contact
Paula Horton
Boeing Communications
1-425-919-9351
[email protected]Boeing Media Relations
[email protected]View original content:https://www.prnewswire.com/news-releases/boeing-airline-partners-set-new-standard-for-parts-authentication-302581242.html
SOURCE Boeing
Continue Reading
-
Rana Muhammad Iqbal and Mansha Ullah Butt to join Punjab cabinet
LAHORE: The Punjab government has approved a fresh expansion of the provincial cabinet, paving the way for former Punjab Assembly Speaker Rana Muhammad Iqbal and senior PML-N leader Mansha Ullah Butt to take oath as ministers today.
The…
Continue Reading
-

YouTube and BBC Studios Laud Content Partnership: “Feed Your Fandom”
YouTube has arrived on the Croisette.
For the first time in the company’s 20-year history, the content behemoth — owned by Google — has come to MIPCOM and used its first keynote session to laud the platform’s partnership with BBC…
Continue Reading