France were up against Azerbaijan in their third game of the UEFA World Cup 2026 Qualifiers. Les Blues had won both their opening games in group D, scoring twice against Ukraine and Iceland.
Didier Deschamps started in a 4-4-2 with Real Madrid’s…

France were up against Azerbaijan in their third game of the UEFA World Cup 2026 Qualifiers. Les Blues had won both their opening games in group D, scoring twice against Ukraine and Iceland.
Didier Deschamps started in a 4-4-2 with Real Madrid’s…

Bigg Boss 19: The weekend ka vaar episode is set to go on air today and bring more drama as the new promo shows Salman Khan schooling contestant Neelam Giri. Khan can be seen asking her what the playground task was all about to Giri and giving a…

Timex has revived one of its most interesting designs from the quartz era: the Q Timex 1975 SSQ Digital Reissue.
The original, launched in the mid-Seventies, was the brand’s first LCD digital watch and one of the earliest models to feature an…
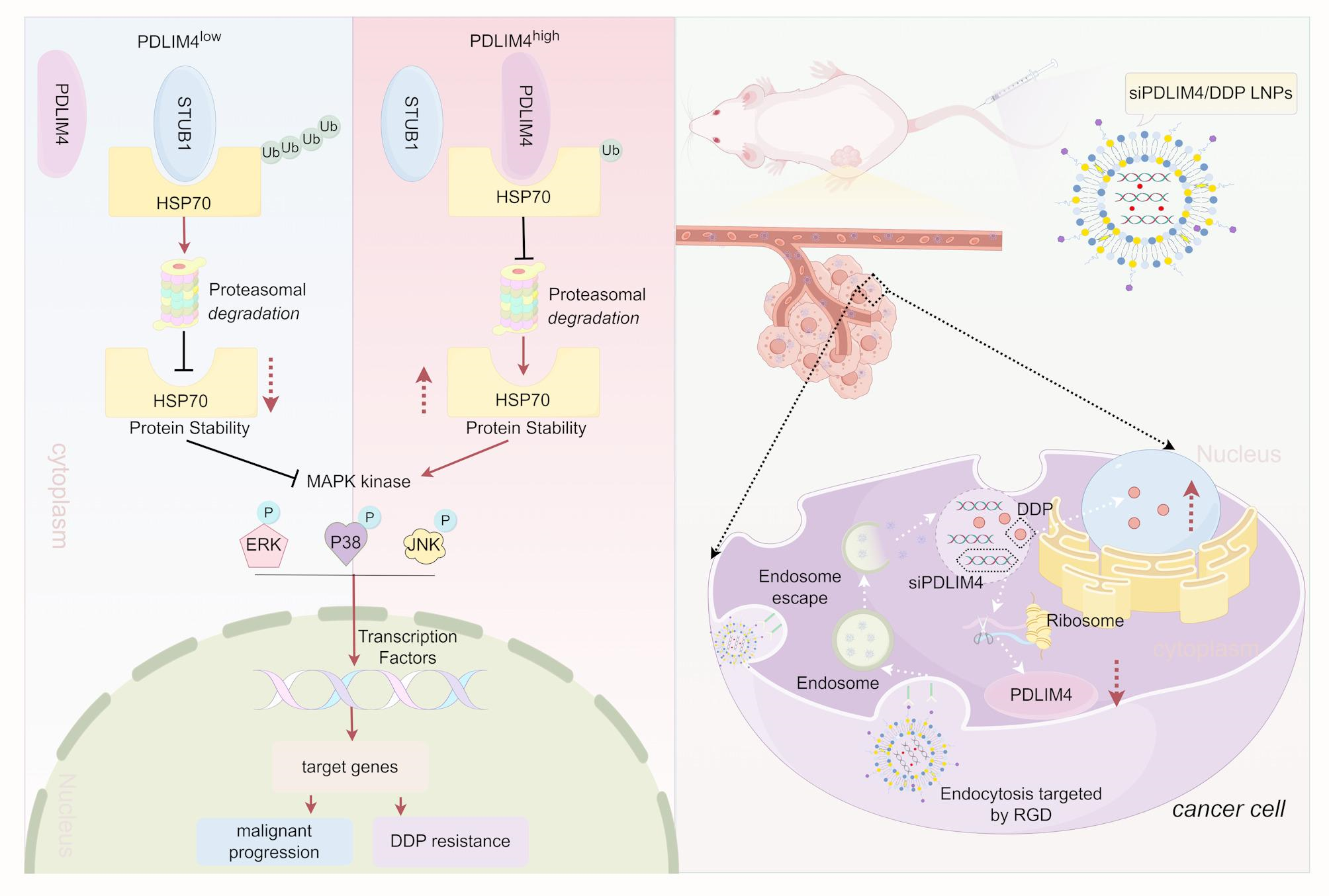
The detailed methods of Cell Lines and Cell Culture, Quantitative Real-Time PCR (RT-qPCR), Cell proliferation, migration assay, Cell survival assay, Cell apoptosis analysis, Identification of Prognosis-Related Genes in Gastric Cancer, Drug Sensitivity Analysis, Stability assay, Cellular Uptake, In vitro Transfection Efficiency are described in Supplementary Experimental Procedures.
A total of 40 pairs of fresh GC tissues and adjacent normal tissues were obtained under the supervision of gastroenterologists. These samples were maintained at − 80 °C to be used in subsequent RT-qPCR and Western blotting analyses, or fixed in 10% formalin and embedded in paraffin for immunohistochemistry (IHC). Additionally, The Pathology Department at the First Affiliated Hospital of Nanchang University provided 210 paraffin-embedded GC tissue samples. All the samples originated from patients diagnosed with GC who underwent surgical procedures between January 2017 and January 2019, none of whom had received any prior treatment. The Ethics Committee of the First Affiliated Hospital of Nanchang University approved the study, and all participants provided informed consent (Approval Number: (2025) CDYFYYLK (04–030)).
General Biol (Anhui) Co., Ltd (Anhui, China) synthesized the siRNA and shRNA primers, with their sequences listed in Table S1. A segment of SFB, whose sequence is also listed in Table S1, was fused to the N-terminus of PDLIM4 to facilitate binding with S-Protein Agarose and Flag antibody. This construct was inserted into the PCDH-CMV-MCS-EF1-copGFP-T2A-Puro vector, resulting in the SFB-PDLIM4 construct. Variants of SFB-PDLIM4, including SFB-PDLIM4 (330AA), SFB-PDLIM4 (1-88AA), SFB-PDLIM4 (1-260AA), SFB-PDLIM4 (80-260AA), SFB-PDLIM4 (80-330AA), and SFB-PDLIM4 (250-330AA) were procured from PPL (Public Protein/Plasmid Library, China). The HSP70 variants, HSP70-ΔN, HSP70-ΔC, and HSP70-ΔABD were procured from LuoRui Biotech (Jiangsu, China). For siRNA and plasmid transfections, Lipo3000 (Thermo Fisher Scientific, USA) and jet PRIME® (Polyplus, France) were employed, respectively. To generate stable cell lines with either knockdown or overexpression of PDLIM4, we utilized psPAX2, pMD2.G, and the target plasmid for lentiviral packaging. The resulting viral supernatant was then used to infect MKN45 and HGC27 cells.
Total Proteins were split by SDS-PAGE, afterwards shifted to 0.45 μm nitrocellulose membranes (Millipore, USA). The membranes were incubated overnight with the primary antibody following 2 h block using 5% milk. Membranes were exposed to a secondary antibody the next day. Image acquisition was conducted using the UVP/ChemStudio Imaging System (Analytik Jena AG, Germany). The antibodies used included PDLIM4 (Abcam, Cat# ab251701), HSP70 (Proteintech, Cat# 10995–1-AP), STUB1 (Abmart, Cat# PHM9480), β-actin (ABclonal, Cat# AC026), GAPDH (Proteintech, Cat# 81640–5-RR), MYC (Proteintech, Cat# 16286–1-AP), Ub (Proteintech, Cat# 10201– 2-AP), HA (ABclonal, Cat# AE008), HA (Proteintech, Cat# 51064–2-AP), DYKDDDDK (Proteintech, Cat# 20543–1-AP), DYKDDDDK (Proteintech, Cat# 66008–4-Ig), P21 (ABclonal, Cat# A19094), P27 (ABclonal, Cat# A19095), bcl2 (Servicebio, Cat# GB154380), Bax (Servicebio, Cat# GB11690), E-cadherin (Proteintech, Cat# 20874–1-AP), MMP2 (Wanleibio, Cat# WL03224), MMP3 (Proteintech, Cat# 66338–1-Ig), MMP9 (Huabio, Cat# ET174-69), Vimentin (Huabio, Cat# ET1610-39), ERK (Proteintech, Cat# 11257–1-AP), P38 (Proteintech, Cat# 66234–1-Ig), JNK (Proteintech, Cat# 66210–1-Ig), p-ERK (Cohesion, Cat# CQA8093), p-P38 (Proteintech, Cat# 28796–1-AP), p-JNK1/2/3 (Cohesion, Cat# CQA8172), Integrin Alpha V + Beta3 (absin, Cat# abs122318), Integrin Alpha V + Beta3 (LMAI, Cat# LM-1310R), Rabbit IgG (Proteintech, Cat# 30000–0-AP) and Mouse IgG (Proteintech, Cat# B900620).
The paraffin-embedded sections underwent deparaffinization and rehydration, antigen retrieval was performed with EDTA (pH 9.0). Subsequently, they were immersed in a 3% hydrogen peroxide solution for 15 min. Sections were incubated overnight at 4 °C with primary antibodies after 2 h block with 5% milk, including PDLIM4 (Abmart, Cat# PC6093M), HSP70 (Proteintech, Cat# 10995–1-AP), and Ki67 (Huabio, Cat# HA721115). The next day, the sections were treated with a secondary antibody for 1 h, then stained with DAB and counterstained with hematoxylin. A scoring system was employed to evaluate the degree of staining, assessing the proportion of positive cells using the following scale: 0 (0%), 1 (1–24% positive cells), 2 (25–49% positive cells), 3 (50–74% positive cells), and 4 (≥ 75% positive cells). The intensity of staining was rated as 0 for negative, 1 for weak, 2 for moderate, and 3 for strong. The immune reactivity score was obtained by multiplying the degree score with the intensity score, with the median value used to differentiate between low and high expression.
MKN45 and HGC27 cells, transfected with SFB-PDLIM4 and HSP70-HA, were fixed using 4% paraformaldehyde for a duration of 30 min. Following this, 0.2% Triton X-100 was used to permeabilize the cells for 15 min. Following permeabilization, the cells were blocked with 5% goat serum for 1 h. Primary antibodies were used to incubate the cells overnight at 4 °C, and the next day, secondary antibodies were applied for 1 h. Imaging was conducted utilizing a ZEISS confocal laser scanning microscope (Germany).
The designated plasmids were used to transfect the cells, which were then lysed using IP lysis buffer (Servicebio, China) containing 50 × Cocktail (Servicebio, China). The cell lysates underwent incubation at 4 °C overnight for co-immunoprecipitation using specified antibody in conjunction with S-Protein Agarose (Millipore, USA) or Protein A/G Plus Sepharose 4FF Affinity Chromatography Resin (Sangon Biotech, China). The beads were washed with TBS buffer at least five times the next day. The final products were boiled in 2 × SDS-PAGE loading buffer (Solarbio, China) at 100 °C for 10 min. The immunoprecipitated samples were analyzed via western blotting.
The PLA was performed using a NaveniFlex Cell Red kit (Navinci, Sweden). On the first day, gastric cancer cells were seeded onto slides, and on the second day, they were fixed and permeabilized. After blocking non-specific binding with Naveni Block, the samples were incubated overnight with primary antibodies (rabbit anti-PDLIM4 and mouse anti-HSP70 (Proteintech, Cat#66183–1-Ig) or mouse anti-IgG). Navenibody were applied and incubated after 1 × TBST washing, with subsequent ligation and amplification steps. Finally, we cleaned the slides and performed nuclear staining using DAPI (Beyotime, China), and took photos using a ZEISS confocal laser scanning microscope (Germany).
The animal research received approval from the Ethics Committee of the First Affiliated Hospital of Nanchang University (Ethics Number: CDYFY-IACUC-202309QR019). Hangzhou Ziyuan Laboratory Animal Technology Co., Ltd. supplied female BALB/c nude mice that were four weeks old. For the mouse xenograft tumor assay, nude mice received a subcutaneous injection of about 5 million cells, and the tumor volume was measured every three days. The calculated using the formula: volume = length × width^2 × 0.5. The mice were euthanized when the tumors grew to a size of 15 mm in diameter, and the tumors were either embedded in paraffin or kept at −80 °C.
Lipid nanoparticles (LNPs) were created by dissolving MC3, DSPC, DMG-PEG2000, cholesterol, and DSPE-PEG-RGD in ethanol with a molar ratio of 50:10:1.5:38.5:1. Subsequently, the lipid mixture and siRNA were combined at a nitrogen-to-phosphate (N/P) ratio of 6, which prepare LNPs encapsulating siPDLIM4 or/and DDP using a microfluidic cartridge.
The size and zeta potential of the nanoparticles were measured using the Zetasizer Nano ZS90 (Malvern Instruments, UK). Transmission electron microscopy (TEM, Japan) was utilized to analyze the nanoparticles’ morphological characteristics. The encapsulation efficiency of siPDLIM4 was assessed using ultraviolet–visible spectroscopy (UV–Vis), while the encapsulation efficiency of DDP was assessed using inductively coupled plasma optical emission spectrometry (ICP-OES). Firstly, we used ICP-OES: Agilent 5110 to detect the Pt content, which is converted to 1 μg/ml Pt≈1.538 μg/ml DDP. We diluted the siPDIM4/DDP LNPs 50 times and measured the average concentration of Pt is 3.786 mg/l, so the Pt concentration in the siPDIM4/DDP LNPs is 189.3 mg/l, which is equivalent to DDP concentration of 291.16 mg/l. We synthesized the siPDIM4/DDP LNPs a total of 10 ml, therefore the content of DDP is 2.9116 mg. 5 mg DDP were used in our synthetic material, the encapsulation efficiency of DDP is 2.9116 mg/5 mg = 58.23%. The concentration of the siPDIM4/DDP LNPs is 3 mg/ml and the volume is 10 ml, therefore, the DDP loading capacity = encapsulated DDP mass/encapsulated DDP mass + carrier mass (2.9116 mg/2.9116 mg + 30 mg = 8.85%).
We conducted a drug release curve in PH6.0 PBS, which is a tumor-mimicking environments. We used the siPDIM4/DDP LNPs with a volume of 1 ml and a concentration of 3 mg/ml for drug release. Then we put it into a dialysis bag, the dialysis solution was 30 ml PBS buffer with pH 6.0. Later, we took out 1 ml dialysis solution at regular intervals and measured the content of Pt released by ICP-OES: Agilent 5110, and converted it to DDP content. The cumulative release = release DDP amount/total DDP amount.
Healthy female mice were assigned into 3 groups randomly (n = 3), with every group receiving a tail vein injection of either naked siPDLIM4, or siPDLIM4 LNPs, siPDLIM4/DDP LNPs, each at a dose of 1 nmol siRNA. The orbital vein was used to collect blood samples using heparinized tubes at predetermined intervals. The fluorescence intensity of CY5-labeled siPDLIM4 in the blood was calculated using a microplate reader to assess pharmacokinetics. For the biodistribution study, healthy female BALB/c nude mice with subcutaneous tumors were randomly divided into two groups (n = 16), with each group receiving an intravenous injection of either naked Cy5-siPDLIM4 or Cy5-siPDLIM4/DDP LNPs. After 1, 6, 24 h, the fluorescence intensity of CY5-siPDLIM4 in major organs and tumors was measured using the small animal CT/live imaging all-in-one machine (Milabs B.V.).
In the mouse xenograft tumor assay, nude mice received subcutaneous injections of approximately 5 million MKN45 cells. Then, on the sixth day the mice of xenograft tumors were divided into five groups (n = 6) through random allocation. On the seventh day, the mice received injections via the tail vein (This injection method refers to relevant literatures [26, 29, 30, 33, 34]) with either PBS, LNPs, siPDLIM4 LNPs, DDP LNPs, or siPDLIM4/DDP LNPs with 1 nmol siRNA or/and DDP dosage of 2 mg/kg, administered every three days for a total of six cycles, which primarily informed by existing literatures [35,36,37]. On the 25th day, the mice were executed, and their major organs and tumors were harvested and preserved in 4% paraformaldehyde for IHC and HE staining. Servicebio (Wuhan, China) conducted an analysis of blood biochemical parameters such as alanine aminotransferase (ALT), aspartate aminotransferase (AST), urea, and creatinine (Crea).
The Cancer Genome Atlas (TCGA) database (http://cancergenome.nih.gov/) provided us with clinical information and RNA-seq transcriptomic data on GC. Additionally, four datasets—GSE66229, GSE122401, GSE179252, and GSE184336 were derived from the Gene Expression Omnibus (GEO) database (http://www.ncbi.nlm.nih.gov/geo/). The GDSC2 drug sensitivity data were accessed from the Genomics of Drug Sensitivity in Cancer (GDSC) database via GitHub (https://github.com/maese005/oncoPredict/tree/main/vignettes). Samples lacking complete clinical information were deemed ineligible and subsequently excluded from the study. Following the preprocessing of raw data, which encompassed sample deduplication, data normalization, probe annotation, gene filtering, and clinical grouping. To analyze differential gene expression between tumor and normal tissues, we used the Lima package in R.
We used R and GraphPad Prism 9.0 for performing statistical analyses. Results are displayed as the mean ± standard deviation based on three different experiments. For differences between two groups, a t-test was utilized, whereas two-way or one-way ANOVA was employed for comparisons among multiple groups. Spearman’s rank order correlation was used to perform the correlation analysis. A P-value below 0.05 was regarded as a sign of statistical significance.
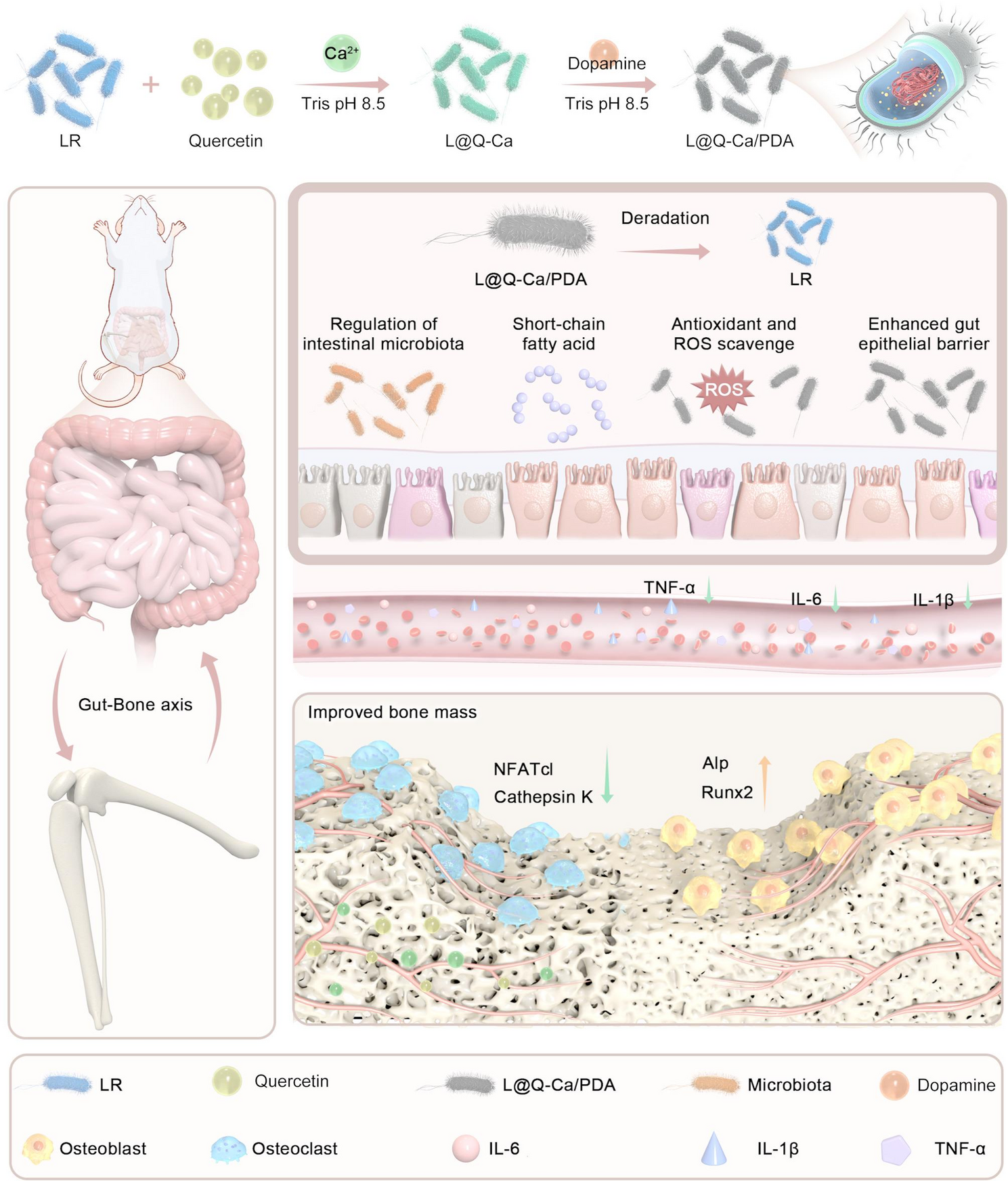
In this study, a layer-by-layer assembly approach was adopted to form a surface nanocoating on probiotics. During the synthesis process, unlike the uncoated LR, the coated LR turned yellowish-green after the MPN coating and black after the polydopamine (PDA) coating (Figure S1A). To investigate the effects of the coating on the growth and proliferation of probiotics, experiments were conducted using single-layer structures of different concentrations of quercetin and dopamine (Figure S1B-C). We found that as the concentration increased, the time for the coating structure to slow the proliferation of probiotics increased, possibly because the nanocoating acts as a rigid barrier, preventing energy exchange between bacteria and the outside world. To further investigate the rationality of the selected concentrations, we explored the rationality of combinations of quercetin and DA at different concentrations. As shown in Figure S1D, as the concentrations of quercetin and dopamine increased, the ROS-scavenging capacity of the probiotics gradually increased. Therefore, we chose a concentration of 0.5 mg/ml for both quercetin and dopamine for subsequent experiments. As shown in Fig. 2A, the probiotic group experienced a 4–6 h delay in exponential growth. As depicted in Fig. 2B, transmission electron microscopy (TEM) images indicated that, compared with the uncoated LR, the LR treated with L@Q-Ca/PDA exhibited a recognizable network coating on its surface. Scanning electron microscopy (SEM) revealed that L@Q-Ca/PDA had a distinctive surface different from that of LR (Figure S1E). Energy dispersive X-ray spectroscopy (EDX) results revealed that C, N, O, and Ca were uniformly distributed on the surface of the probiotics (Fig. 2C). The UV‒Vis absorption spectrum demonstrated that the spectrum of L@Q-Ca/PDA contained characteristic peaks corresponding to LR (254–265 nm), PDA (280–300 nm), and quercetin (271 nm) (Fig. 2D). Fourier transform infrared spectroscopy also showed distinct peaks, with 3410 cm-1 (-OH) and 1611 cm-1 corresponding to quercetin and 1499 cm-1 (C-H) corresponding to dopamine (Fig. 2E). The presence of these peaks in the spectrum of L@Q-Ca/PDA further confirmed the presence of the double coating. Dynamic light scattering (DLS) measurements indicated that both the size and zeta potential changed after the nanocoating process (Fig. 2F). The zeta potential values showed that the charge of LR became more negative after being wrapped by MPN, which was consistent with the finding that the polyphenol-based complex has a net negative charge. After the MPN coating was added, the negative potential increased from − 11.6 to −14.1 mV, and after the PDA coating was added, it increased from − 14.1 to −12.2 mV. These changes in zeta potential confirmed that the LR cells were successfully encapsulated by the first MPN layer and the subsequent PDA layer. Moreover, as the nanocoating progressed, the size of the LR gradually increased. As shown in Fig. 2G and Figure S1F, confocal laser scanning microscopy (CLSM) revealed that the green fluorescence of the MPN overlapped with the red fluorescence of the rhodamine B-labeled PDA. These results collectively confirmed the successful formation of a continuous shell on individual microorganisms.
Preparation and characterization of L@Q-Ca/PDA. (A) Growth curves of LR, L@Q-Ca, L@Q-Ca/PDA; (B) Representative TEM images of LR, L@Q-Ca, L@Q-Ca/PDA; (C) HAADF-STEM image and corresponding elemental mapping images of C, N, O, and Ca; (D) UV–vis absorption spectra of LR, L@Q-Ca, L@Q-Ca/PDA; (E) Fourier transform infrared spectra of LR, L@Q-Ca, L@Q-Ca/PDA; (F) Zeta potentials and size distributions of the samples measured by DLS; (G) Typical confocal images of L@Q-Ca/PDA. Scale bar: 1.2 μm. The red channel shows rhodamine B-labeled MPNs and the green channel indicates PDA-incorporated coatings
The ability of bacteria to tolerate various extreme environments is crucial for their survival and subsequent colonization in the gastrointestinal tract. Before they reach the intestine and begin colonization, they must survive in the gastrointestinal environment. Therefore, we evaluated the protective effects of various coatings on probiotics in the gastrointestinal environment. As shown in Fig. 3A, to further confirm the protective effect of the coating, the probiotics were incubated in SGF for 4 h and then in SIF for 6 h. As illustrated in Fig. 3B and Figure S2A, the survival of LR was affected by acidic environments, but not all of them died. After incubation with SGF for 1 h, the survival rate of LR was relatively high. After incubation with SGF for 2 h, the survival rate of LR decreased. After incubation with SGF for 4 h, the survival rate of LR significantly decreased. However, the survival rate of LR after coating significantly increased. As shown in Fig. 3C and Figure S2B, similar phenomena were observed in the bile salt environment. When the incubation time with bile salt was extended from 1 h to 4 h, the survival rate of coated LR was significantly greater than that of uncoated LR. As shown in Fig. 3D and Figure S2C, in the SIF environment, all the LR groups had relatively high survival rates. However, the survival rate of the uncoated LR was greater, which might have been affected by the multilayer nanostructure. As shown in Figure S2D and S2E, in the H2O2 environment, L@Q-Ca/PDA also increased the survival rate. These findings indicate that the MPN and PDA coatings can effectively protect probiotics and increase their survival rate. TEM (Fig. 3E) confirmed that the morphology of the uncoated LR changed, while the morphology of the coated bacteria remained intact, accompanied by the dissolution and shedding of the outer shell. Live/dead bacterial staining (Figure S2F) revealed that the L@Q-Ca/PDA coating significantly increased the survival rate of the probiotics. Through plate counting (Figure S2G), it was observed that the number of surviving coated LR was greater than that of uncoated LR after incubation with SGF for 4 h and SIF for 6 h. This further indicates that the coating can further enhance the ability of LR to resist the chemical environment of the gastrointestinal tract.
Probiotics must withstand the harsh chemical environment of the gastrointestinal tract and successfully colonize and proliferate within the intestinal tract. The ability to adhere to the intestinal mucosa is a basic prerequisite for successful colonization and a key factor for the continuous treatment of intestinal inflammatory sites. To clarify the enhanced adhesion of the L@Q-Ca/PDA multilayer coating to LR in the intestine, we used cell models and animal models for verification. In the cell model, Caco-2 cells were selected because of their widespread application in simulating human intestinal epithelial cells in vitro. CLSM imaging was used to visualize the colocalization of green fluorescence (FITC-labeled Caco-2 cells) and red fluorescence (rhodamine B-labeled probiotics). The coated bacteria exhibited considerably stronger red fluorescence signals than the uncoated bacteria (Fig. 3F).
The successful colonization of probiotics is closely related to their long-term retention in the intestine. In the animal model, an IVIS Spectrum was used to study and compare the retention times of natural LR and L@Q-Ca/PDA in the gastrointestinal tract. As shown in Fig. 3G, in the fluorescence images, the fluorescence signal in the abdomen of the LR mice rapidly decreased after 8 h, whereas the fluorescence signal of L@Q-Ca/PDA decreased significantly slower than that of LR in the first 8 h. Moreover, at 120 h (the 6th day), the fluorescence signal of LR was weak, whereas that of L@Q-Ca/PDA was still strong. After 120 h (Fig. 3H, I), the fluorescence images and quantitative results of the isolated mouse intestines indicated that only a small amount of uncoated LR was found throughout the intestine, mainly with a weak fluorescence signal in the cecum. Similarly, L@Q-Ca showed a moderate fluorescence signal. However, the L@Q-Ca/PDA group exhibited intense fluorescence in the cecum and colon, clearly demonstrating that the multilayer coating of L@Q-Ca/PDA significantly enhanced the long-term adhesion and colonization of probiotics in the cecum and colon. In addition, to investigate the in vivo status of quercetin from orally administered L@Q-Ca/PDA, we measured the blood levels and systemic distribution of quercetin in mice. Within 8 h after oral administration of L@Q-Ca/PDA, the quercetin concentration in the blood gradually increased but then slowly decreased (Figure S2H). The results of in vivo imaging (Figure S2I) revealed that quercetin was most abundant in the liver, followed by the kidneys. We subsequently measured in vitro calcium ion release from L@Q-Ca/PDA at different pH levels and H2O2 concentrations (Figure S2J).
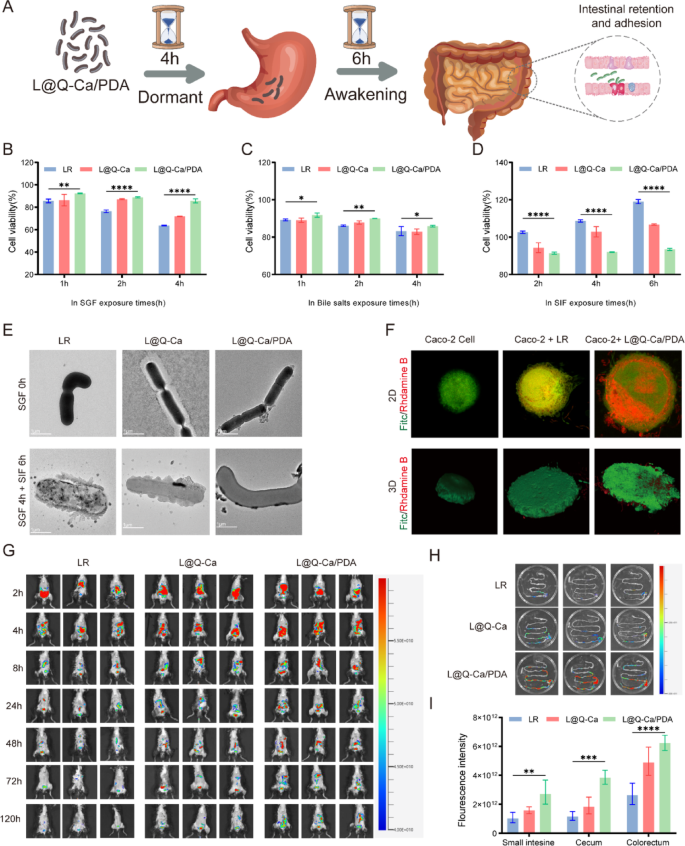
Resistance of L@Q-Ca/PDA Against Environmental Assaults and Mucoadhesive Capability. Probiotics were separately exposed to harsh environments as follows: (A) Schematic illustration of the awakening process; (B) SGF (pH 2.0), (C) bile salt (0.3 mg·mL − 1), and (D) SIF (pH 6.8); (E) TEM images of L@Q-Ca/PDA after incubation in SGF for 0 h, SGF for 4 h and SIF for 6 h, respectively; (F) Adhesion of probiotic strains in each group on Caco-2 cells observed by CLSM. Green: Caco-2 stained with fluorescein isothiocyanate (FITC). Red: rhodamine B-labeled probiotics; (G) Representative IVIS images of mice administered with various rhodamine B-labeled probiotic formulations; (H) IVIS images of mouse gut at 120 h post-administration of various rhodamine B-labeled probiotic formulations; (I) Region-of-interest analysis of fluorescence intensity of the mouse GIT at 120 h. Statistical analysis was performed using one-way ANOVA.*P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001
In the osteoporosis model induced by chronic inflammation, the concentration of reactive oxygen species (ROS) within the intestinal mucosa is notably increased. High concentrations of ROS can reduce the activity of probiotics and hinder their effective colonization in the intestine [24]. Therefore, we evaluated the resistance and scavenging ability of L@Q-Ca/PDA against ROS in vitro (Fig. 4A). We sought to verify the ROS scavenging ability of L@Q-Ca/PDA, as shown in Fig. 4B. Compared with the LR group, L@Q-Ca/PDA significantly inhibited the production of ROS and effectively cleared intracellular ROS, which we attribute to the ability of the bacterial outer coating to scavenge ROS. Additionally, the ability of L@Q-Ca/PDA to mitigate oxidative stress was further verified by detecting the expression levels of GSH, MDA, SOD, and T-AOC, and its ability to alleviate ROS-induced cellular oxidative damage and inflammatory responses was evaluated. The results shown in Fig. 4C-F indicated that L@Q-Ca/PDA could significantly increase the levels of GSH, SOD, and T-AOC while reducing the level of MDA. As shown in Fig. 4G-J, L@Q-Ca/PDA significantly decreased the mRNA expression levels of TNF-α and IL-6 in H2O2-treated Raw264.7 cells and increased the mRNA expression levels of TGF-β and IL-10. Furthermore, the anti-inflammatory ability of L@Q-Ca/PDA was evaluated at the protein level by measuring the expression of CD206 and INOS in Raw264.7 cells. The immunofluorescence results (Fig. 4K) revealed that, compared with that in the LR group, the expression of CD206 in the L@Q-Ca/PDA group was significantly increased, while the expression of INOS was significantly decreased. The Western blot results (Figure S3A-C) further confirmed this phenomenon. All the above results indicated that the ROS-scavenging and anti-inflammatory antioxidant ability of L@Q-Ca/PDA was much greater than that of simple LR. These findings suggest that the nanocoating can enhance the ability of probiotics to resist inflammatory diseases. In addition, through alizarin red and TRAP staining, we evaluated the ability of the coating to promote osteogenesis and inhibit osteoclastogenesis. As shown in Figure S3D-E, compared with the other groups, the L@Q-Ca/PDA group exhibited stronger osteogenic ability. As shown in Figure S3F, L@Q-Ca/PDA significantly inhibited RANKL-induced osteoclastogenesis. The above results demonstrated that the nanocoating could enhance the anti-inflammatory and antioxidant effects of probiotics and increase their capacity to promote osteogenesis and inhibit osteoclastogenesis.
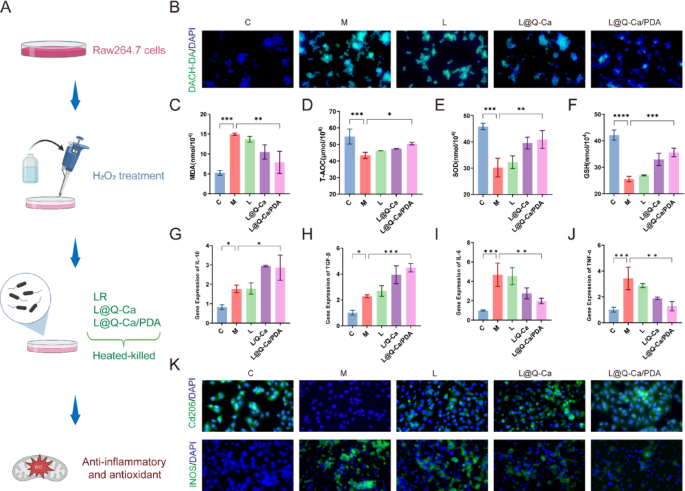
Anti-inflammatory and antioxidant effects of L@Q-Ca/PDA in vitro. (A) Schematic illustration of the experimental design; (B) Intracellular ROS levels of H2O2-treated RAW 264.7 cells after different treatments; Antioxidant-related indices were detected, including MDA (C), T-AOC (D), SOD (E), and GSH (F); (G-J) RT-qPCR analysis of IL-10 (G), TGF-β (H), TNF-α (I), and IL-6 (J) mRNA expression in RAW 264.7 cells after different treatments as indicated; (K) iNOS and CD206 fluorescence images of H2O2-treated RAW 264.7 cells after different treatments. Statistical analysis was performed using one-way ANOVA. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001
To verify whether the encapsulated probiotics enhance survival and adhesion under pathological conditions, we further investigated whether L@Q-Ca/PDA could serve as an enhanced therapeutic approach for osteoporosis induced by chronic inflammation. First, we evaluated the therapeutic effect of L@Q-Ca/PDA on alleviating DSS-induced intestinal inflammatory injury. The experimental procedure is shown in Fig. 5A. The mice were given free access to drinking water containing 2.5% DSS for seven days to induce acute colitis, followed by free access to drinking water containing 1.5% DSS until Day 30. A dose of 2 × 109 CFU of LR, L@Q-Ca or L@Q-Ca/PDA was administered orally every other day for therapeutic purposes. During the continuous 30-day administration period, the consistency of the stool, the presence of bloody stool and the changes in the body weight of the mice were recorded. As shown in Fig. 5B, the weight loss of mice in the L@Q-Ca/PDA treatment group was significantly inhibited. Similarly, the disease activity index (DAI) indicated that the disease activity index of the L@Q-Ca/PDA treatment group was significantly reduced (Fig. 5C). Moreover, L@Q-Ca/PDA also inhibited the shortening of the colon caused by DSS, with the colon length of the L@Q-Ca/PDA group approaching that of the healthy control group (Fig. 5D, E). H&E staining, AB‒PAS staining, and Masson staining of the colon tissue (Fig. 5F-G; Figure S4A) revealed that compared with the PBS treatment, the LR, L@Q-Ca and L@Q-Ca/PDA treatments reversed inflammatory cell infiltration, intestinal mucosal barrier damage and fibrosis caused by DSS to varying degrees, among which the L@Q-Ca/PDA treatment had the best therapeutic effect. As shown in Fig. 5H-I, the TUNEL staining results indicated that L@Q-Ca/PDA significantly inhibited the apoptosis of intestinal epithelial cells. Furthermore, intestinal mucosal barrier function was assessed by immunofluorescence (Fig. 5J-L), which revealed that compared with that in the DSS model group, the expression of the intestinal epithelial tight junction proteins ZO-1 and Occludin significantly increased in the LR, L@Q-Ca and L@Q-Ca/PDA treatment groups. These findings indicate that L@Q-Ca/PDA has good intestinal epithelial repair capabilities. The assessment of serum inflammatory factors (Fig. 5M-O) revealed that the expression of the proinflammatory factors IL-6, IL-1β and TNF-α significantly decreased in the L@Q-Ca/PDA group. Additionally, HE staining of the mice’s heart, liver, spleen, lung, and kidney (Figure S4C) and liver and kidney function (Figure S4D) revealed that this material system is safe.
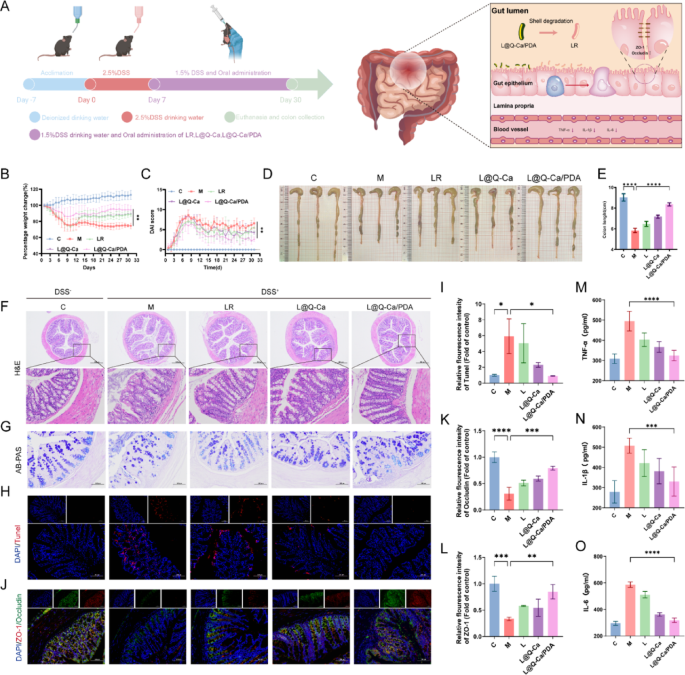
Therapeutic efficacy of L@Q-Ca/PDA against DSS-induced intestinal mucositis. (A) Schematic illustration of modeling and treatment; (B) Percentage change in body weight of mice and (C) DAI scores during the experiment; (D) Colon images, and (E) quantified colon lengths of mice; Representative H&E staining (F) and AB-PAS staining (G) images of the colon tissue; (H) Fluorescence images and quantitative analysis (I) of TUNEL staining in colon tissue; (J) Immunofluorescence images and quantitative analysis of the expression of ZO-1 (K) and Occludin (L) in the colon; Expression of the proinflammatory cytokines TNF-α (M) ,IL-1β (N) and IL-6 (O) in the blood of mice. Statistical analysis was performed using one-way ANOVA. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001.
We subsequently assessed the efficacy of L@Q-Ca/PDA in treating osteoporosis in mice with DSS-induced inflammatory osteoporosis by evaluating the expression of both osteoblast and osteoclast markers (Fig. 6A). Three-dimensional (3D) CT reconstruction images and quantitative analysis of trabecular and cortical bone in the femur revealed that after L@Q-Ca/PDA treatment, the bone density, trabecular bone volume fraction, and trabecular bone number of the mice significantly increased, whereas the trabecular bone spacing decreased (Fig. 6B-C). Compared with that in the LR group, the therapeutic effect of L@Q-Ca/PDA was significantly improved. Moreover, H&E staining and Masson staining were used to evaluate the reconstruction of osteoporotic bone defects. H&E staining (Fig. 6D) revealed that the degree of defects was greatest in the model group, and there was almost no new bone at the edges of the defects. In contrast, the bone defects in the L@Q-Ca/PDA treatment group were significantly reduced compared with those in the other treatment groups, and the volume of the newly formed calluses significantly increased. Masson staining (Fig. 6E-F) revealed that the amounts of newly formed collagen tissue and immature bone tissue significantly increased in the L@Q-Ca/PDA treatment group. OCN staining (Fig. 6G-H) further demonstrated the excellent osteogenic ability of L@Q-Ca/PDA. TRAP staining (Fig. 6I-K) revealed that compared with that in the model group, the number of osteoclasts in the other groups decreased to varying degrees, and the therapeutic effect of L@Q-Ca/PDA was the greatest, with the number of osteoclasts in the L@Q-Ca/PDA treatment group approaching that in the healthy control group. The results of WB experiments (Fig. 6L-M) revealed that compared with those in the other treatment groups, the expression levels of the osteogenic-related proteins Alp and Runx2 in the L@Q-Ca/PDA treatment group significantly increased, while the expression levels of the osteoclast-related proteins Cathepsin K and NFAc1 significantly decreased. These findings further highlight the excellent anti-osteoporotic effect of L@Q-Ca/PDA in chronic inflammation-induced osteoporosis.
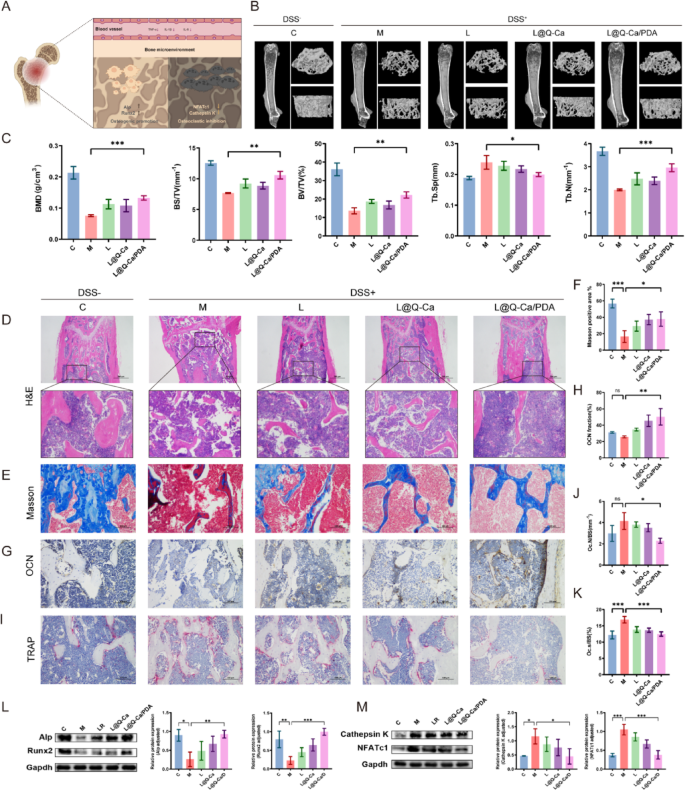
Therapeutic efficacy of L@Q-Ca/PDA in osteoporosis in model mice with colitis-induced osteoporosis. (A) Schematic illustration of systemic inflammation relief in osteoporosis; (B) Micro-CT images of the distal femur in different groups; (C) BMD, BV/TV, BS/TV, Tb.N, and Tb.Sp structural parameters in different groups of DSS-induced colitis mice; Representative H&E staining (D), Masson staining (E, F), OCN staining (G, H), and TRAP staining (I-K) images of bone tissue and their quantitative results; (L) Western blotting analysis of osteogenic factors (RUNX2 and ALP) with protein levels and quantitative results; (M) Western blotting analysis of osteoclast factors (Cathepsin K and NFATc1) with protein levels and quantitative results; Statistical analysis was performed using one-way ANOVA. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001
Furthermore, the therapeutic efficacy of L@Q-Ca/PDA in an ovariectomized (OVX) osteoporosis model was rigorously confirmed (Fig. 7A). As shown in Fig. 7B, treatment with L@Q-Ca/PDA significantly inhibited the reduction in uterine weight caused by ovariectomy but had a minor effect on the body weight of the mice (Fig. 7C). As depicted in Fig. 7D and E, in the OVX mouse model, three-dimensional (3D) CT reconstruction images and quantitative analysis of the femur revealed that compared with those of the model group mice, the L@Q-Ca/PDA treatment group showed significant increases in bone density, trabecular bone volume fraction, and trabecular bone number, while the trabecular bone spacing was significantly reduced. H&E, Masson, TRAP, and OCN staining of the femur all indicated that the treatments with LR, L@Q-Ca, and L@Q-Ca/PDA to varying degrees increased osteoblast activity and decreased osteoclast activity, reducing bone loss caused by ovariectomy, with the therapeutic effect of L@Q-Ca/PDA being the most significant (Fig. 7F-M). In addition, we explored changes in the mouse gut in a mouse model of ovariectomized osteoporosis. HE, AB‒PAS and TUNEL staining (Fig. 7N-Q) revealed no significant differences among the groups, but the expression of ZO-1 and Occludin increased significantly in the L@Q-Ca/PDA group (Fig. 7R-T). These findings further suggest that L@Q-Ca/PDA can strengthen the intestinal mucosal barrier. The WB (Fig. 7U-V) revealed that, compared with those in the model group, the expression levels of the osteogenic proteins Alp and Runx2 were significantly increased in the L@Q-Ca/PDA group, whereas the expression levels of the osteoclastic proteins Cathepsin K and NFATc1 were significantly decreased. These results further confirmed that, consistent with the results of the chronic inflammation-induced osteoporosis model, L@Q-Ca/PDA still had a significant therapeutic effect on the ovariectomized osteoporosis mouse model. Additionally, the results of HE staining of various organs (Figure S4E) and liver and kidney function tests (Figure S4F) further confirmed the safety of this material system.
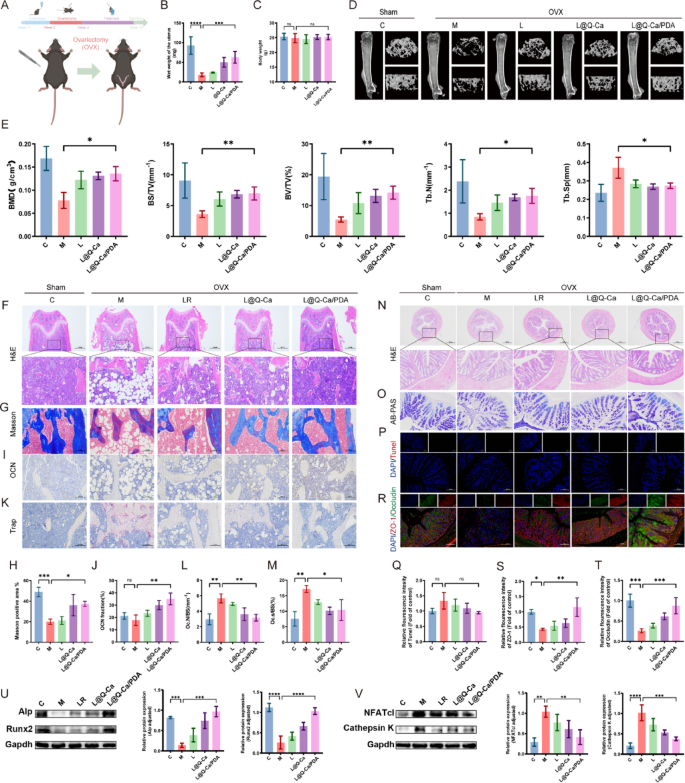
Therapeutic efficacy of L@Q-Ca/PDA in model mice with OVX Osteoporosis. (A) Schematic illustration of modeling and treatment; (B) Wet weight of the uterus and (C) body weight of mice during the experiment; (D) Micro-CT images of the distal femur in different groups and (E) their quantitative results; Representative H&E staining (F), Masson staining (G, H), OCN staining (I, J), and TRAP staining (K, L, M) images of bone tissue and their relative quantitative results; Representative H&E staining (N), AB-PAS staining (O), TUNEL staining (P, Q), and ZO-1/Occludin staining (R, S, T) images of colon tissue and their relative quantitative results; (U) Western blotting analysis of osteogenic factors (RUNX2 and ALP) with protein levels and quantitative results; (V) Western blotting analysis of osteoclast factors (Cathepsin K and NFATc1) with protein levels and quantitative results; Statistical analysis was performed using one-way ANOVA. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001
Finally, we analyzed the changes in the intestinal microbiota of mice in a DSS-induced inflammatory osteoporosis model and an ovariectomized osteoporosis model. In both osteoporosis models, β-diversity analysis (PCoA) revealed a separation of the intestinal microbiota structure between the control group and the osteoporosis model group, and treatment with L@Q-Ca/PDA altered the microbiota structure of the mice (Fig. 8A and H). In the DSS-induced model, the ASV/OTU Venn diagram revealed 187 shared OTUs among the five groups, and the numbers of unique OTUs in each group were 2247, 3087, 2638, 17,112, and 2196 (Figure S5A). In the estrogen-deficiency-induced model, there were 264 shared OTUs among the five groups, and the numbers of unique OTUs in each group were 1077, 802, 568, 755, and 610 (Figure S6A). We subsequently analyzed the composition of the fecal microbiota in each group of mice. In both models, we observed that at the phylum level, Firmicutes and Bacteroidota were the dominant phyla in the fecal microbiota samples (Fig. 8B and I). At the genus level, Lactobacillus was the most abundant genus in the fecal microbiota, and the relative abundance of Lactobacillus in the L@Q-Ca/PDA group was significantly greater than that in the M group (Fig. 8C, D and J, K). In the inflammatory osteoporosis model, linear discriminant analysis effect size (LEfSe) analysis (Fig. 8E and Figure S5B) indicated that the dominant bacteria in the L@Q-Ca/PDA group were Ligilactobacillus (g), Escherichia (g), Enterobacteriaceae_A (f), and Enterobacterales_A (o). In the estrogen deficiency model, the results of the LEfSe analysis (Fig. 8L and Figure S6B) revealed that the dominant bacteria in the L@Q-Ca/PDA group were Chloroflexi (p), Porphyromonadaceae (f), Parabacteroides (g), Luteimonas (g), Brachybacterium (g), Dermabacteraceae (f), Rikenella (g), Dietzia (g), and Rhizobiales (o). We subsequently used targeted metabolomics to measure the levels of SCFAs in feces. The results showed that L@Q-Ca/PDA significantly changed the levels of SCFAs in the feces of mice in both models, especially by significantly increasing the level of acetic acid (Fig. 8F, G and M, N). Additionally, in the inflammatory osteoporosis model, the level of butyric acid was significantly increased (Figure S5C). Moreover, the levels of other types of SCFAs in each intervention group also changed (Figure S5D-H and S6C-H). These results indicate that L@Q-Ca/PDA significantly altered the composition and levels of the fecal microbiota and SCFAs in both osteoporosis models, especially affecting Lactobacillus and acetic acid.
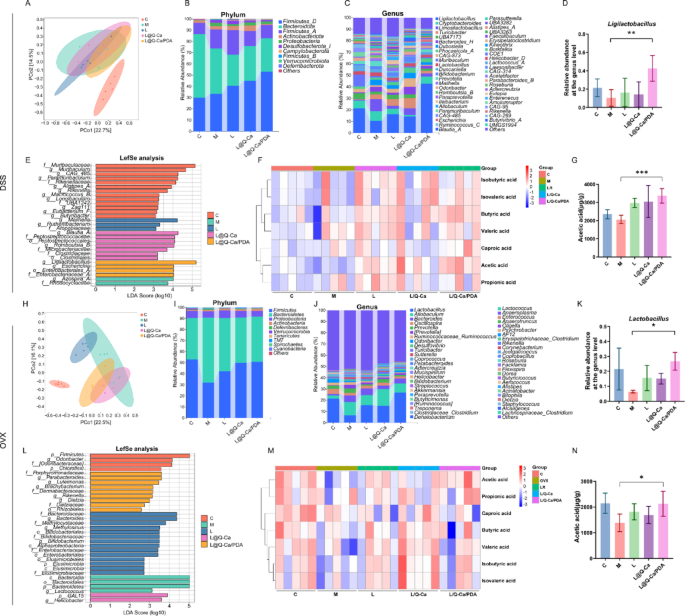
Regulation of L@Q-Ca/PDA on gut microbiome homeostasis in mouse models of colitis-induced osteoporosis and OVX-induced osteoporosis. (A, H) PCoA score plots illustrating the β-diversity of the gut microbiome; (B, I) Relative abundances of gut commensal microbes at the phyla levels after different treatments; (C, J) Relative abundances of gut microbiota at the genus level; (D, K) Relative contents of Lactobacillus in fecal samples; (E, L) Distribution histograms based on linear discriminant analysis (LDA). LDA scores higher than 2.0 indicate taxa that are significantly more abundant in the corresponding group than in other groups; (F, M) Heatmap illustrations of short-chain fatty acids; (G, N) Relative contents of acetic acid in fecal samples; Statistical analysis was performed using Student’s t-test. * P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001
Osteoporosis is a prevalent systemic bone disease that remains a significant clinical challenge because of the limitations of existing therapies, including side effects and incomplete targeting of underlying pathological mechanisms [25]. This study aims to overcome the key obstacles to oral probiotic therapy for osteoporosis, namely, low bacterial survival rates and poor efficacy, by developing a multifunctional nanocoated probiotic system (L@Q-Ca/PDA), that can enhance gastrointestinal tolerance and synergistically regulate the gut–bone axis. Our key findings indicate that this nanoarmor-assisted probiotic strategy significantly improves the survival rate of probiotics in the harsh gastrointestinal environment, alleviates intestinal dysregulation, and effectively treats osteoporosis in inflammatory and estrogen-deficient mouse models by coordinately regulating intestinal inflammation, oxidative stress, barrier integrity, and intestinal flora homeostasis.
The structural design of L@Q-Ca/PDA represents a rational integration of materials science and microbiology to overcome the limitations of probiotics. Through various characterization techniques such as TEM, DLS, and FTIR, it was confirmed that quercetin and calcium ions successfully formed a metal‒phenol network (MPN), which was then covered with a PDA layer. This double-layer structure has two key advantages: First, the outer PDA layer acts as a robust physical barrier to protect LR from gastric acid, bile salts, and digestive enzymes. In agreement with the findings of previous studies, the rigid nanocoating barrier prolongs the proliferation cycle of probiotics [26]. PDA has strong adhesion and redox activity and can buffer strongly acidic environments [27]; the calcium‒polyphenol network can protect probiotics and enhance their anti-inflammatory and antioxidant effects [28]. Second, the inner MPN layer not only enhances structural stability but also provides a reservoir of quercetin, which can be gradually released in the intestine to exert anti-inflammatory and bone-protective effects. The delayed exponential growth of coated probiotics (Fig. 2A) indicates minimal impairment of bacterial metabolic activity, ensuring their functional integrity after delivery—a key improvement over rigid nanocarriers, which often impair microbial viability [18].
The enhanced gastrointestinal survival rate of L@Q-Ca/PDA directly translates to superior biological effects. In vitro experiments revealed that the coated probiotics exhibited strong resistance to oxidative stress (H₂O₂) and bile salts, and live–dead staining confirmed that their survival rate was higher than that of uncoated LR. This protective effect was accompanied by enhanced anti-inflammatory activity, as manifested by a reduction in proinflammatory cytokines (IL-6 and TNF-α) and an increase in anti-inflammatory mediators (IL-10 and TGF-β) in RAW264.7 cells, which may be mediated by the combined effect of the antioxidant properties of PDA and the inhibition of NF-κB signaling by quercetin [29, 30]. Importantly, L@Q-Ca/PDA also promoted osteoblast differentiation (by upregulating Alp and Runx2) and inhibited osteoclast formation (by downregulating Cathepsin K and NFATc1), which is consistent with the known role of quercetin in balancing bone remodeling and the ability of LR to regulate bone–immune crosstalk. Furthermore, previous studies have shown that calcium ions can synergistically promote osteogenic differentiation and mineralization processes with the aforementioned components through the calmodulin signaling pathway [31]. In summary, L@Q-Ca/PDA improves the osteoporotic microenvironment in multiple ways, such as by inhibiting inflammation and regulating bone metabolism through the synergistic effect of multiple components, and is expected to ultimately achieve effective alleviation of osteoporosis.
In vivo, the therapeutic effect of L@Q-Ca/PDA was verified in two different osteoporosis models. In the DSS-induced inflammatory osteoporosis model, L@Q-Ca/PDA reversed weight loss, reduced the DAI, and restored colon length (an indicator of intestinal recovery), with better effects than uncoated LR or single-layer-coated L@Q-Ca did. Histological analysis revealed improved intestinal barrier integrity (upregulated expression of ZO-1 and Occludin) and reduced epithelial apoptosis, which is consistent with reports that quercetin can repair barrier damage [22] and that the mucosal adhesion properties of PDA enhance local retention [32]. In the OVX model, compared with OVX, L@Q-Ca/PDA alleviated uterine atrophy and increased BMD, and micro-CT revealed that the trabecular structure was restored—these effects were better than those of traditional probiotic therapy [33]. These results confirm that the gut–bone axis is a viable therapeutic target and extend previous observations that intestinal flora imbalance leads to bone loss [34].
A key mechanistic insight is that L@Q-Ca/PDA functions by regulating the intestinal flora. In both models, 16 S rDNA sequencing revealed significant enrichment of Lactobacillus; changes in the Firmicutes/Bacteroidota ratio; and a concomitant increase in short-chain fatty acids (SCFAs), especially acetic acid. SCFAs regulate osteoclast activity through GPR43 signaling [35] and enhance intestinal barrier function [36], which provides a reasonable link between changes in intestinal microorganisms and improvements in bone health. The unique microbial characteristics induced by L@Q-Ca/PDA (such as Ligilactobacillus in the DSS model and Parabacteroides in the OVX model) indicate its background-dependent flora remodeling ability, highlighting the adaptability of the system to osteoporosis caused by different pathological triggers.
Compared with existing strategies, our method has three key innovations. First, unlike monofunctional probiotic preparations [17] or chemical drugs [1], L@Q-Ca/PDA combines the advantages of live probiotics, natural antioxidants (quercetin), and protective nanocoatings to achieve synergistic effects on the intestine and bones. Second, the degradability of MPN and PDA negates the risk of long-term accumulation, solving the safety problems associated with nonbiodegradable nanocarriers [11]. Third, the oral administration route is clinically translatable, overcoming the limitations of injectable osteoporosis therapies [5]. Although previous studies have used PDA coatings for drug delivery [37] or MPN for microbial protection [26], the integration of these layers with probiotics to target the gut–bone axis is novel and fills a gap that currently exists in the field of osteoporosis management.
The clinical significance of this work is significant. Current osteoporosis treatments rely on antiresorptive drugs that pose a risk of osteonecrosis (such as bisphosphonates) or on high-cost anabolic drugs. L@Q-Ca/PDA utilizes natural compounds and probiotics with clear safety profiles, providing a safe and low-cost alternative. The system is effective in both inflammatory and estrogen-deficient models, indicating its applicability to both primary and secondary osteoporosis. Future directions include optimizing the coating thickness for controlled release, testing in large animal models, and exploring combinations with existing drugs to enhance efficacy. In addition, mechanistic studies on SCFA-mediated bone signaling and flora–bone crosstalk may reveal new therapeutic targets.
This study has several limitations. First, although mouse models are widely used, they may not fully reproduce human osteoporosis, especially in terms of disease progression and intestinal flora complexity. Second, the molecular mechanism linking increased SCFA levels to improved bone mineral density requires more detailed research. Third, the optimal in vivo administration regimen of L@Q-Ca/PDA and the grouping of animal experiments warrant optimization. Future studies need to supplement comparative experiments with different concentration combinations to optimize the formulation and provide a more accurate basis for clinical dose selection.
In conclusion, this study proposes a multifunctional nanocoated probiotic system that treats osteoporosis by increasing the gastrointestinal survival rate of the bacteria and synergistically regulating the gut–bone axis. By overcoming the key limitations of probiotic therapy and offering mechanistic insights into gut–bone crosstalk, this strategy paves the way for a novel and clinically translatable approach to osteoporosis management.

Most corporate laptop fleets consist primarily of PCs. However, there’s always a contingent of users who beg for Macs. Deciding who gets a Mac in your organization involves balancing IT’s need for simplicity, finance’s requirement to keep…

Experts have found that siblings can shape how young birds learn critical life skills – and in some cases, they may matter more than parents.
A new field study from the University of California, Davis, and the Max Planck Institute of Animal…

In yet…

A GROUNDBREAKING study has provided new evidence that vitamin C in pregnancy may influence childhood lung function by altering DNA methylation at key asthma- and allergy-associated loci, particularly in cases where mothers smoke during…

How did you get into comedy?
I was on maternity leave and, looking back, I may have been having a manic episode. I’d had a long string of admin jobs that I hated. Usually, it was the case that I didn’t know what my job was and nobody else did…