Jack DeJohnette, the jazz drummer celebrated as one of the genre’s true greats – who worked with stars including Miles Davis, Sonny Rollins and Charles Lloyd – has died aged 83. A press representative for ECM, the record label that released…
Blog
-

AI restores James Webb telescope’s crystal-clear vision
Two PhD students from Sydney have helped restore the sharp vision of the world’s most powerful space observatory without ever leaving the ground. Louis Desdoigts, now a postdoctoral researcher at Leiden University in the Netherlands, and his…
Continue Reading
-
iPhone 18 Pro variable aperture camera rumor surfaces once again – AppleInsider
- iPhone 18 Pro variable aperture camera rumor surfaces once again AppleInsider
- iPhone 18 Pro Could Gain DSLR Camera-Style Aperture Control MacRumors
- Apple’s A20 chips could launch a new era of thinner, cooler iPhones AppleInsider
- Apple A20 And A20…
Continue Reading
-

London Marathon winners Assefa and Sawe headline World Athletics Out of Stadium Athlete of the Year nominees
How to vote for World Athletics Out of Stadium Athlete of the Year 2025
Fans can vote for their Women’s and Men’s Out of Stadium Athlete of the Year on World Athletics’ social media pages, with each individual ‘like’ on Facebook or Instagram and…
Continue Reading
-
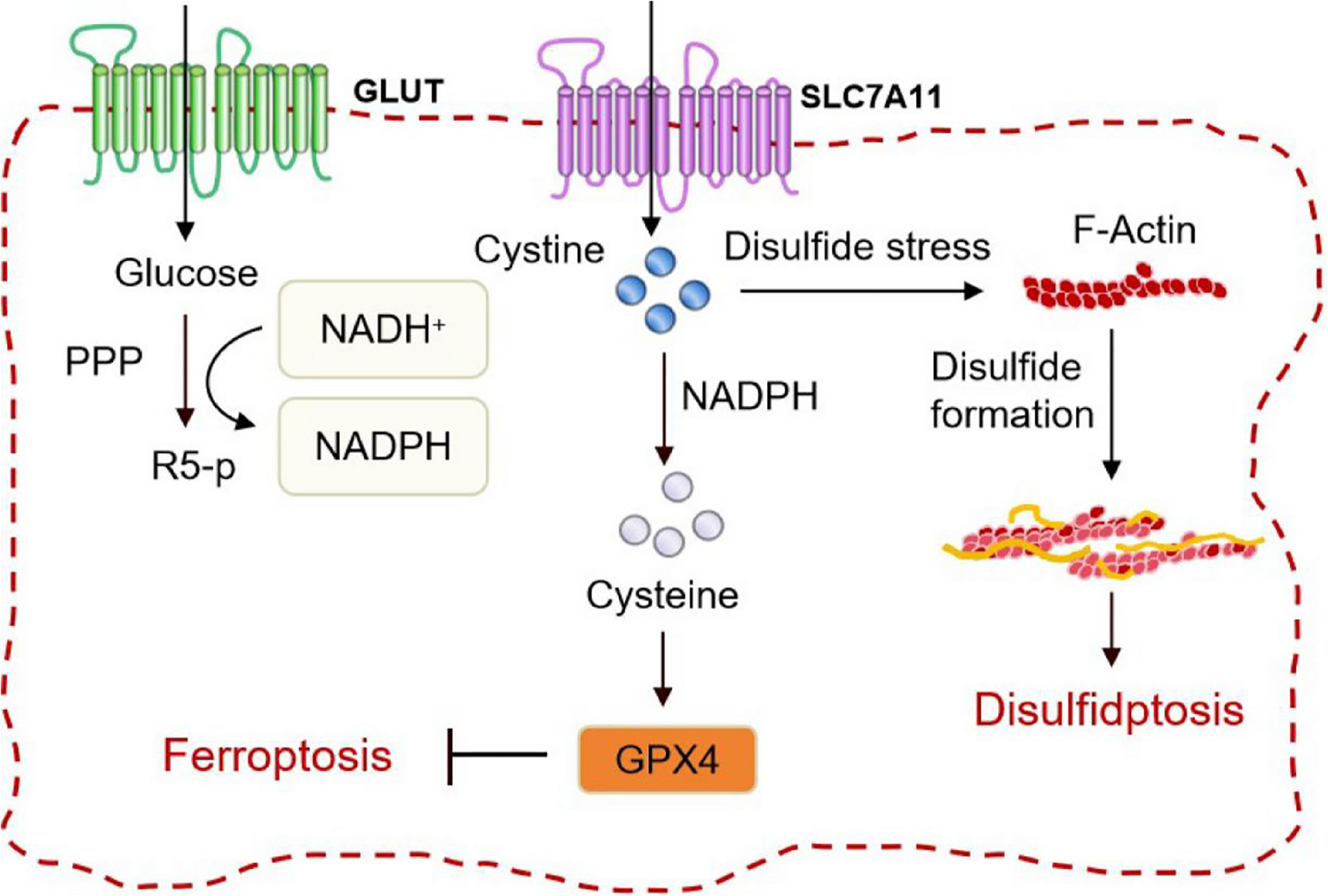
SLC7A11 as a bridge between ferroptosis and disulfidptosis: a promising target for tumor treatment | Cell Communication and Signaling
Newton K, Strasser A, Kayagaki N, Dixit VM. Cell death. Cell. 2024;187(2):235–56. https://doi.org/10.1016/j.cell.2023.11.044.
Google Scholar
Green DR, Victor B. The pantheon of the fallen: why are there so many forms of cell death? Trends Cell Biol. 2012;22(11):555–6. https://doi.org/10.1016/j.tcb.2012.08.008.
Google Scholar
Dixon SJ, Lemberg KM, Lamprecht MR, et al. Ferroptosis: an iron-dependent form of nonapoptotic cell death. Cell. 2012;149(5):1060–72. https://doi.org/10.1016/j.cell.2012.03.042.
Google Scholar
Bannai S, Kitamura E. Transport interaction of L-cystine and L-glutamate in human diploid fibroblasts in culture. J Biol Chem. 1980;255(6):2372–6.
Google Scholar
Fotiadis D, Kanai Y, Palacín M. The SLC3 and SLC7 families of amino acid transporters. Mol Aspects Med. 2013;34(2–3):139–58. https://doi.org/10.1016/j.mam.2012.10.007.
Google Scholar
Koppula P, Zhang Y, Zhuang L, Gan B. Amino acid transporter SLC7A11/xCT at the crossroads of regulating redox homeostasis and nutrient dependency of cancer. Cancer Commun (Lond). 2018;38(1):12. https://doi.org/10.1186/s40880-018-0288-x.
Google Scholar
Mou Y, Wang J, Wu J, He D, Zhang C, Duan C, et al. Ferroptosis, a new form of cell death: opportunities and challenges in cancer. J Hematol Oncol. 2019;12(1):34. https://doi.org/10.1186/s13045-019-0720-y.
Google Scholar
Liu J, Xia X, Huang P, xCT:. A critical molecule that links cancer metabolism to redox signaling. Mol Ther. 2020;28(11):2358–66. https://doi.org/10.1016/j.ymthe.2020.08.021.
Google Scholar
Koppula P, Zhuang L, Gan B. Cystine transporter SLC7A11/xCT in cancer: ferroptosis, nutrient dependency, and cancer therapy. Protein Cell. 2021;12(8):599–620. https://doi.org/10.1007/s13238-020-00789-5.
Google Scholar
Liu X, Nie L, Zhang Y, et al. Actin cytoskeleton vulnerability to disulfide stress mediates Disulfidptosis. Nat Cell Biol. 2023;25(3):404–14. https://doi.org/10.1038/s41556-023-01091-2.
Google Scholar
Lee N, Park SJ, Lange M, et al. Selenium reduction of ubiquinone via SQOR suppresses ferroptosis. Nat Metab. 2024;6(2):343–58. https://doi.org/10.1038/s42255-024-00974-4.
Google Scholar
Jiang X, Stockwell BR, Conrad M. Ferroptosis: mechanisms, biology and role in disease. Nat Rev Mol Cell Biol. 2021;22(4):266–82. https://doi.org/10.1038/s41580-020-00324-8.
Google Scholar
Lin Q, Zhou H, Zeng J, et al. Bioactive polysaccharides mediate ferroptosis to modulate tumor immunotherapy. Int J Biol Macromol. 2024. https://doi.org/10.1016/j.ijbiomac.2024.135147.
Google Scholar
Mao C, Wang M, Zhuang L, Gan B. Metabolic cell death in cancer: ferroptosis, cuproptosis, disulfidptosis, and beyond. Protein Cell. 2024;15(9):642–60. https://doi.org/10.1093/procel/pwae003.
Google Scholar
Chen X, Kang R, Kroemer G, Tang D. Broadening horizons: the role of ferroptosis in cancer. Nat Rev Clin Oncol. 2021;18(5):280–96. https://doi.org/10.1038/s41571-020-00462-0.
Google Scholar
Tang D, Chen X, Kang R, Kroemer G. Ferroptosis: molecular mechanisms and health implications. Cell Res. 2021;31(2):107–25. https://doi.org/10.1038/s41422-020-00441-1.
Google Scholar
Tong X, Tang R, Xiao M, et al. Targeting cell death pathways for cancer therapy: recent developments in necroptosis, pyroptosis, ferroptosis, and Cuproptosis research. J Hematol Oncol. 2022;15(1):174. https://doi.org/10.1186/s13045-022-01392-3.
Google Scholar
Lang X, Green MD, Wang W, et al. Radiotherapy and immunotherapy promote tumoral lipid oxidation and ferroptosis via synergistic repression of SLC7A11. Cancer Discov. 2019;9(12):1673–85. https://doi.org/10.1158/2159-8290.CD-19-0338.
Google Scholar
Li Y, Yan J, Zhao Q, Zhang Y, Zhang Y. ATF3 promotes ferroptosis in sorafenib-induced cardiotoxicity by suppressing Slc7a11 expression. Front Pharmacol. 2022;13:904314. https://doi.org/10.3389/fphar.2022.904314.
Google Scholar
Bassi MT, Gasol E, Manzoni M, et al. Identification and characterisation of human xCT that co-expresses, with 4F2 heavy chain, the amino acid transport activity system Xc-. Pflugers Arch. 2001;442(2):286–96. https://doi.org/10.1007/s004240100537.
Google Scholar
Bridges CC, Kekuda R, Wang H, et al. Structure, function, and regulation of human cystine/glutamate transporter in retinal pigment epithelial cells. Invest Ophthalmol Vis Sci. 2001;42(1):47–54.
Google Scholar
Kim JY, Kanai Y, Chairoungdua A, et al. Human cystine/glutamate transporter: cDNA cloning and upregulation by oxidative stress in glioma cells. Biochim Biophys Acta. 2001;1512(2):335–44.
Google Scholar
Sato H, Tamba M, Kuriyama-Matsumura K, Okuno S, Bannai S. Molecular cloning and expression of human xCT, the light chain of amino acid transport system Xc-. Antioxid Redox Signal. 2000;2(4):665–71. https://doi.org/10.1089/ars.2000.2.4-665.
Google Scholar
Li S, Lu Z, Sun R, et al. The role of SLC7A11 in cancer: friend or foe? Cancers (Basel). 2022;14(13):3059. https://doi.org/10.3390/cancers14133059.
Google Scholar
Yang J, Zhou Y, Xie S, et al. Metformin induces ferroptosis by inhibiting ufmylation of SLC7A11 in breast cancer. J Exp Clin Cancer Res. 2021;40(1):206. https://doi.org/10.1186/s13046-021-02012-7.
Google Scholar
Badgley MA, Kremer DM, Maurer HC, et al. Cysteine depletion induces pancreatic tumor ferroptosis in mice. Science. 2020;368(6486):85–9. https://doi.org/10.1126/science.aaw9872.
Google Scholar
Hong T, Lei G, Chen X, et al. PARP inhibition promotes ferroptosis via repressing SLC7A11 and synergizes with ferroptosis inducers in BRCA-proficient ovarian cancer. Redox Biol. 2021;42:101928. https://doi.org/10.1016/j.redox.2021.101928.
Google Scholar
Park JW, Kilic O, Deo M, et al. CIC reduces xCT/SLC7A11 expression and glutamate release in glioma. Acta Neuropathol Commun. 2023;11(1):13. https://doi.org/10.1186/s40478-023-01507-y.
Google Scholar
Long Y, Tao H, Karachi A, et al. Dysregulation of glutamate transport enhances Treg function that promotes VEGF blockade resistance in glioblastoma. Cancer Res. 2020;80(3):499–509. https://doi.org/10.1158/0008-5472.CAN-19-1577.
Google Scholar
Yuan L, Li S, Chen Q, et al. EBV infection-induced GPX4 promotes chemoresistance and tumor progression in nasopharyngeal carcinoma. Cell Death Differ. 2022;29(8):1513–27. https://doi.org/10.1038/s41418-022-00939-8.
Google Scholar
Combs JA, DeNicola GM. The non-essential amino acid cysteine becomes essential for tumor proliferation and survival. Cancers (Basel). 2019;11(5):678. https://doi.org/10.3390/cancers11050678.
Google Scholar
Carlisle AE, Lee N, Matthew-Onabanjo AN, et al. Selenium detoxification is required for cancer-cell survival. Nat Metab. 2020;2(7):603–11. https://doi.org/10.1038/s42255-020-0224-7.
Google Scholar
Lee N, Carlisle AE, Kim D. Examining xCT-mediated selenium uptake and Selenoprotein production capacity in cells. Methods Enzymol. 2022;662:1–24. https://doi.org/10.1016/bs.mie.2021.10.002.
Google Scholar
Goji T, Takahara K, Negishi M, Katoh H. Cystine uptake through the cystine/glutamate antiporter xCT triggers glioblastoma cell death under glucose deprivation. J Biol Chem. 2017;292(48):19721–32. https://doi.org/10.1074/jbc.M117.814392.
Google Scholar
Timmerman LA, Holton T, Yuneva M, et al. Glutamine sensitivity analysis identifies the xCT antiporter as a common triple-negative breast tumor therapeutic target. Cancer Cell. 2013;24(4):450–65. https://doi.org/10.1016/j.ccr.2013.08.020.
Google Scholar
Shin CS, Mishra P, Watrous JD, et al. The glutamate/cystine xCT antiporter antagonizes glutamine metabolism and reduces nutrient flexibility. Nat Commun. 2017;8:15074. https://doi.org/10.1038/ncomms15074.
Google Scholar
Romero R, Sayin VI, Davidson SM, et al. Keap1 loss promotes Kras-driven lung cancer and results in dependence on glutaminolysis. Nat Med. 2017;23(11):1362–8. https://doi.org/10.1038/nm.4407.
Google Scholar
Bannai S. Induction of cystine and glutamate transport activity in human fibroblasts by diethyl maleate and other electrophilic agents. J Biol Chem. 1984;259(4):2435–40.
Google Scholar
Bannai S, Kitamura E. Adaptive enhancement of cystine and glutamate uptake in human diploid fibroblasts in culture. Biochim Biophys Acta. 1982;721(1):1–10. https://doi.org/10.1016/0167-4889(82)90017-9.
Google Scholar
Bannai S, Sato H, Ishii T, Taketani S. Enhancement of glutathione levels in mouse peritoneal macrophages by sodium arsenite, cadmium chloride and glucose/glucose oxidase. Biochim Biophys Acta. 1991;1092(2):175–9. https://doi.org/10.1016/0167-4889(91)90153-o.
Google Scholar
Pakos-Zebrucka K, Koryga I, Mnich K, Ljujic M, Samali A, Gorman AM. The integrated stress response. EMBO Rep. 2016;17(10):1374–95. https://doi.org/10.15252/embr.201642195.
Google Scholar
Ye P, Mimura J, Okada T, et al. Nrf2- and ATF4-dependent upregulation of xCT modulates the sensitivity of T24 bladder carcinoma cells to proteasome inhibition. Mol Cell Biol. 2014;34(18):3421–34. https://doi.org/10.1128/MCB.00221-14.
Google Scholar
Ye Y, Chen A, Li L, et al. Repression of the antiporter SLC7A11/glutathione/glutathione peroxidase 4 axis drives ferroptosis of vascular smooth muscle cells to facilitate vascular calcification. Kidney Int. 2022;102(6):1259–75. https://doi.org/10.1016/j.kint.2022.07.034.
Google Scholar
Wang L, Liu Y, Du T, et al. ATF3 promotes erastin-induced ferroptosis by suppressing system Xc. Cell Death Differ. 2020;27(2):662–75. https://doi.org/10.1038/s41418-019-0380-z.
Google Scholar
Zhao X, Chen C, Qiu H, et al. The landscape of ATF3 in tumors: metabolism, expression regulation, therapy approach, and open concerns. Pharmacol Res. 2025;214:107666. https://doi.org/10.1016/j.phrs.2025.107666.
Google Scholar
Wang Y, Yang L, Zhang X, et al. Epigenetic regulation of ferroptosis by H2B monoubiquitination and p53. EMBO Rep. 2019;20(7):e47563. https://doi.org/10.15252/embr.201847563.
Google Scholar
Zhang Y, Koppula P, Gan B. Regulation of H2A ubiquitination and SLC7A11 expression by BAP1 and PRC1. Cell Cycle. 2019;18(8):773–83. https://doi.org/10.1080/15384101.2019.1597506.
Google Scholar
Tsuchihashi K, Okazaki S, Ohmura M, et al. The EGF receptor promotes the malignant potential of glioma by regulating amino acid transport system xc(-). Cancer Res. 2016;76(10):2954–63. https://doi.org/10.1158/0008-5472.CAN-15-2121.
Google Scholar
Liu L, He J, Sun G, et al. The N6-methyladenosine modification enhances ferroptosis resistance through inhibiting SLC7A11 mRNA deadenylation in hepatoblastoma. Clin Transl Med. 2022;12(5):e778. https://doi.org/10.1002/ctm2.778.
Google Scholar
Zhu Y, Zhang C, Huang M, Lin J, Fan X, Ni T. TRIM26 induces ferroptosis to inhibit hepatic stellate cell activation and mitigate liver fibrosis through mediating SLC7A11 ubiquitination. Front Cell Dev Biol. 2021;9:644901. https://doi.org/10.3389/fcell.2021.644901.
Google Scholar
Li S, Lu Z, Sun R, et al. The role of SLC7A11 in cancer: friend or foe? Cancers (Basel). 2022;14(13):3059. https://doi.org/10.3390/cancers14133059.
Google Scholar
Ishimoto T, Nagano O, Yae T, et al. CD44 variant regulates redox status in cancer cells by stabilizing the xCT subunit of system xc(-) and thereby promotes tumor growth. Cancer Cell. 2011;19(3):387–400. https://doi.org/10.1016/j.ccr.2011.01.038.
Google Scholar
Gan W, Dai X, Dai X, et al. LATS suppresses mTORC1 activity to directly coordinate Hippo and mTORC1 pathways in growth control. Nat Cell Biol. 2020;22(2):246–56. https://doi.org/10.1038/s41556-020-0463-6.
Google Scholar
Zhang W, Feng J, Ni Y, et al. The role of SLC7A11 in diabetic wound healing: novel insights and new therapeutic strategies. Front Immunol. 2024;15:1467531. https://doi.org/10.3389/fimmu.2024.1467531.
Google Scholar
Gu Y, Albuquerque CP, Braas D, et al. mTORC2 regulates amino acid metabolism in cancer by phosphorylation of the Cystine-Glutamate antiporter xCT. Mol Cell. 2017;67(1):128–e1387. https://doi.org/10.1016/j.molcel.2017.05.030.
Google Scholar
Stockwell BR, Friedmann Angeli JP, Bayir H, et al. Ferroptosis: a regulated cell death nexus linking metabolism, redox biology, and disease. Cell. 2017;171(2):273–85. https://doi.org/10.1016/j.cell.2017.09.021.
Google Scholar
Doll S, Proneth B, Tyurina YY, et al. ACSL4 dictates ferroptosis sensitivity by shaping cellular lipid composition. Nat Chem Biol. 2017;13(1):91–8. https://doi.org/10.1038/nchembio.2239.
Google Scholar
Kagan VE, Mao G, Qu F, et al. Oxidized arachidonic and adrenic PEs navigate cells to ferroptosis. Nat Chem Biol. 2017;13(1):81–90. https://doi.org/10.1038/nchembio.2238.
Google Scholar
Chu B, Kon N, Chen D, et al. ALOX12 is required for p53-mediated tumour suppression through a distinct ferroptosis pathway. Nat Cell Biol. 2019;21(5):579–91. https://doi.org/10.1038/s41556-019-0305-6.
Google Scholar
Jiang L, Kon N, Li T, et al. Ferroptosis as a p53-mediated activity during tumour suppression. Nature. 2015;520(7545):57–62. https://doi.org/10.1038/nature14344.
Google Scholar
Jennis M, Kung CP, Basu S, et al. An African-specific polymorphism in the TP53 gene impairs p53 tumor suppressor function in a mouse model. Genes Dev. 2016;30(8):918–30. https://doi.org/10.1101/gad.275891.115.
Google Scholar
Zhang Y, Shi J, Liu X, et al. BAP1 links metabolic regulation of ferroptosis to tumour suppression. Nat Cell Biol. 2018;20(10):1181–92. https://doi.org/10.1038/s41556-018-0178-0.
Google Scholar
Liu T, Jiang L, Tavana O, Gu W. The deubiquitylase OTUB1 mediates ferroptosis via stabilization of SLC7A11. Cancer Res. 2019;79(8):1913–24. https://doi.org/10.1158/0008-5472.CAN-18-3037.
Google Scholar
Reck M, Carbone DP, Garassino M, Barlesi F. Targeting KRAS in non-small-cell lung cancer: recent progress and new approaches. Ann Oncol. 2021;32(9):1101–10. https://doi.org/10.1016/j.annonc.2021.06.001.
Google Scholar
Xiong HJ, Yu HQ, Zhang J, et al. Elevated FBXL6 activates both wild-type KRAS and mutant KRASG12D and drives HCC tumorigenesis via the ERK/mTOR/PRELID2/ROS axis in mice. Mil Med Res. 2023;10(1):68. https://doi.org/10.1186/s40779-023-00501-8.
Google Scholar
Mueller S, Engleitner T, Maresch R, et al. Evolutionary routes and KRAS dosage define pancreatic cancer phenotypes. Nature. 2018;554(7690):62–8. https://doi.org/10.1038/nature25459.
Google Scholar
Hu K, Li K, Lv J, et al. Suppression of the SLC7A11/glutathione axis causes synthetic lethality in KRAS-mutant lung adenocarcinoma. J Clin Invest. 2020;130(4):1752–66. https://doi.org/10.1172/JCI124049.
Google Scholar
Lim JKM, Delaidelli A, Minaker SW, et al. Cystine/glutamate antiporter xCT (SLC7A11) facilitates oncogenic RAS transformation by preserving intracellular redox balance. Proc Natl Acad Sci U S A. 2019;116(19):9433–42. https://doi.org/10.1073/pnas.1821323116.
Google Scholar
Chen X, Li J, Kang R, Klionsky DJ, Tang D. Ferroptosis: machinery and regulation. Autophagy. 2021;17(9):2054–81. https://doi.org/10.1080/15548627.2020.1810918.
Google Scholar
Wang W, Green M, Choi JE, et al. CD8 + T cells regulate tumour ferroptosis during cancer immunotherapy. Nature. 2019;569(7755):270–4. https://doi.org/10.1038/s41586-019-1170-y.
Google Scholar
Kim DH, Kim WD, Kim SK, Moon DH, Lee SJ. TGF-β1-mediated repression of SLC7A11 drives vulnerability to GPX4 Inhibition in hepatocellular carcinoma cells. Cell Death Dis. 2020;11(5):406. https://doi.org/10.1038/s41419-020-2618-6.
Google Scholar
Dai E, Han L, Liu J, et al. Autophagy-dependent ferroptosis drives tumor-associated macrophage polarization via release and uptake of oncogenic KRAS protein. Autophagy. 2020;16(11):2069–83. https://doi.org/10.1080/15548627.2020.1714209.
Google Scholar
Bi G, Liang J, Zhao M, et al. MiR-6077 promotes cisplatin/pemetrexed resistance in lung adenocarcinoma via CDKN1A/cell cycle arrest and KEAP1/ferroptosis pathways. Mol Ther. 2022;28:366–86. https://doi.org/10.1016/j.omtn.2022.03.020.
Google Scholar
Qin K, Zhang F, Wang H, et al. CircRNA circSnx12 confers cisplatin chemoresistance to ovarian cancer by inhibiting ferroptosis through a miR-194-5p/SLC7A11 axis. BMB Rep. 2023;56(2):184–9. https://doi.org/10.5483/BMBRep.2022-0175.
Google Scholar
Sun C, Liu P, Pei L, Zhao M, Huang Y. Propofol inhibits proliferation and augments the anti-tumor effect of doxorubicin and paclitaxel partly through promoting ferroptosis in triple-negative breast cancer cells. Front Oncol. 2022;12:837974. https://doi.org/10.3389/fonc.2022.837974.
Google Scholar
Yadav P, Sharma P, Sundaram S, Venkatraman G, Bera AK, Karunagaran D. SLC7A11/ xCT is a target of miR-5096 and its restoration partially rescues miR-5096-mediated ferroptosis and anti-tumor effects in human breast cancer cells. Cancer Lett. 2021;522:211–24. https://doi.org/10.1016/j.canlet.2021.09.033.
Google Scholar
Chen X, Kang R, Kroemer G, Tang D. Ferroptosis in infection, inflammation, and immunity. J Exp Med. 2021;218(6):e20210518. https://doi.org/10.1084/jem.20210518.
Google Scholar
Mi T, Kong X, Chen M, Guo P, He D. Inducing disulfidptosis in tumors: potential pathways and significance. MedComm (2020). 2024;5(11):e791. https://doi.org/10.1002/mco2.791
Yan Y, Teng H, Hang Q, et al. Slc7a11 expression level dictates differential responses to oxidative stress in cancer cells. Nat Commun. 2023;14(1):3673. https://doi.org/10.1038/s41467-023-39401-9.
Google Scholar
Joly JH, Delfarah A, Phung PS, Parrish S, Graham NA. A synthetic lethal drug combination mimics glucose deprivation-induced cancer cell death in the presence of glucose. J Biol Chem. 2020;295(5):1350–65. https://doi.org/10.1074/jbc.RA119.011471.
Google Scholar
Liu T, Ren Y, Wang Q, et al. Exploring the role of the disulfidptosis-related gene SLC7A11 in adrenocortical carcinoma: implications for prognosis, immune infiltration, and therapeutic strategies. Cancer Cell Int. 2023;23(1):259. https://doi.org/10.1186/s12935-023-03091-6.
Google Scholar
Zhao D, Meng Y, Dian Y, et al. Molecular landmarks of tumor disulfidptosis across cancer types to promote disulfidptosis-target therapy. Redox Biol. 2023;68:102966. https://doi.org/10.1016/j.redox.2023.102966.
Google Scholar
Li J, Yu T, Sun J, et al. Integrated analysis of disulfidptosis-related immune genes signature to boost the efficacy of prognostic prediction in gastric cancer. Cancer Cell Int. 2024;24(1):112. https://doi.org/10.1186/s12935-024-03294-5.
Google Scholar
Xia Q, Yan Q, Wang Z, et al. Disulfidptosis-associated lncRNAs predict breast cancer subtypes. Sci Rep. 2023;13(1):16268. https://doi.org/10.1038/s41598-023-43414-1.
Google Scholar
Liu L, Liu J, Lyu Q, et al. Disulfidptosis-associated lncRNAs index predicts prognosis and chemotherapy drugs sensitivity in cervical cancer. Sci Rep. 2023;13(1):12470. https://doi.org/10.1038/s41598-023-39669-3.
Google Scholar
Korangath P, Teo WW, Sadik H, et al. Targeting glutamine metabolism in breast cancer with aminooxyacetate. Clin Cancer Res. 2015;21(14):3263–73. https://doi.org/10.1158/1078-0432.CCR-14-1200.
Google Scholar
Stine ZE, Schug ZT, Salvino JM, Dang CV. Targeting cancer metabolism in the era of precision oncology. Nat Rev Drug Discov. 2022;21(2):141-162. doi:10.1038/s41573-021-00339-6
Shao N, Qiu H, Liu J, et al. Targeting lipid metabolism of macrophages: a new strategy for tumor therapy. J Adv Res. 2025;68:99–114. https://doi.org/10.1016/j.jare.2024.02.009.
Google Scholar
Zhao X, Zhao J, Li D, et al. Akkermansia muciniphila: a potential target and pending issues for oncotherapy. Pharmacol Res. 2023;196:106916. https://doi.org/10.1016/j.phrs.2023.106916.
Google Scholar
Liu X, Zhuang L, Gan B. Disulfidptosis: disulfide stress-induced cell death. Trends Cell Biol. 2024;34(4):327–37. https://doi.org/10.1016/j.tcb.2023.07.009.
Google Scholar
Qiu H, Shao N, Liu J, et al. Amino acid metabolism in tumor: new shine in the fog? Clin Nutr. 2023;42(8):1521–30. https://doi.org/10.1016/j.clnu.2023.06.011.
Google Scholar
Liu J, Shao N, Qiu H, et al. Intestinal microbiota: A Bridge between intermittent fasting and tumors. Biomed Pharmacother. 2023;167:115484. https://doi.org/10.1016/j.biopha.2023.115484.
Google Scholar
Continue Reading
-
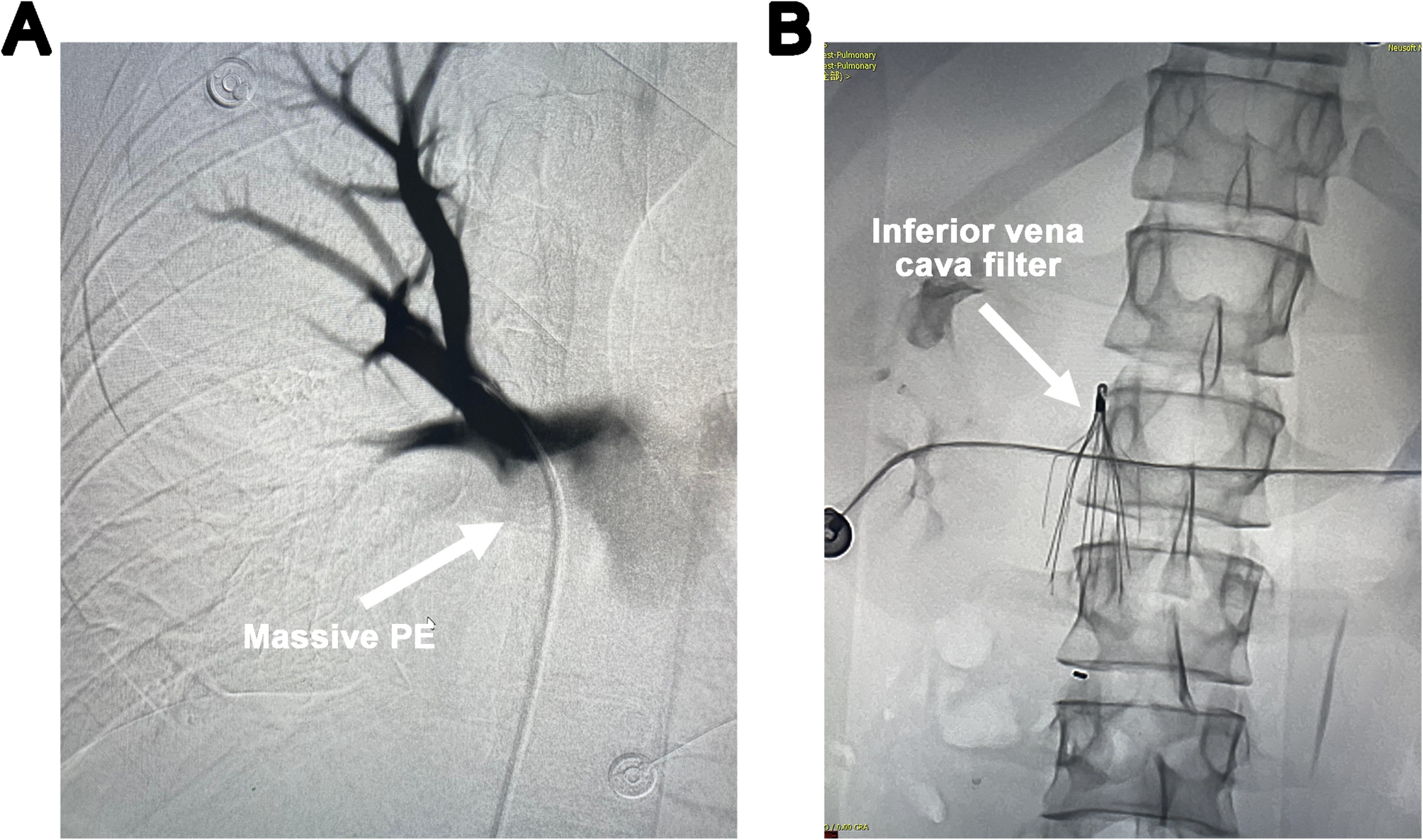
Inserting a venoarterial extracorporeal membrane oxygenation drainage cannula into the inferior vena cava through the internal jugular vein in a patient with an inferior vena cava filter | International Journal of Emergency Medicine
An 18-year-old male patient, a Moroccan international student in China, presented to the emergency department of Union Hospital, Tongji Medical College, Huazhong University of Science and Technology, with a chief complaint of persistent upper…
Continue Reading
-

These teams that have qualified for the IFAF Flag Football World Championships 2026
The fields are now set for the women’s and men’s competitions at the 2026 IFAF Flag Football World Championships.
Following the conclusion of the Asia-Oceania Flag Football Championships on Sunday (26 October), the final continental competition…
Continue Reading
-

AI Helps Decode The Epigenetic ‘off-switch’ In An Ugly Plant That Lives For 3,000 Years
We all age, and for decades, science has been looking for a way to slow that clock. Much of that research is now focused on epigenetics—a system of “software” that runs on our genetic “hardware,” telling genes when to turn on and off.…
Continue Reading
-

Sotheby’s Records Surrealist Records in Paris—and More Art News
The Headlines
SUSPECTED LOUVRE THIEVES ARRESTED. Two suspects have been detained in connection with the theft of jewels, including pieces once belonging to Emperor Napoleon III and Empress Eugénie, from the Louvre on October 19….
Continue Reading
-

Eli Lilly buys Adverum in eye disease gene therapy punt
As part of the deal, Eli Lilly will acquire Adverum’s lead candidate ixo-vec, an intravitreal gene therapy for wAMD treatment. Credit: Tada Images via Shutterstock.com. 
Eli Lilly has agreed to acquire eye disease specialist Adverum Biotechnologies, bucking a recent trend of big pharma companies deciding to steer clear of the cell and gene therapy sector.
Eli Lilly has offered Adverum $3.56 per share in cash, including an additional $8.91 in milestone payments. The latter depends on US approval of the biotech’s lead gene therapy candidate, ixo-vec, within seven years and achieving more than $1bn in annual global sales within ten years. This brings the total consideration to $12.47 a share, valuing the deal at a possible $261.7m.
Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
Find out more
The share offer agreed on 24 October reflects a nearly 15% discount from the $4.18 closing price on 23 October.
For Adverum, the potential buyout from Eli Lilly provides financial respite. The biotech has been struggling for cash in recent times – holding $44.4m to its name in July 2025. The lack of capital had increased jeopardy for ixo-vec, an intravitreal gene therapy that advanced into a Phase III trial (NCT06856577) for the treatment of wet age-related macular degeneration (wAMD) in March 2025.
Indeed, Eli Lilly stated that without a $65m loan given to Adverum to continue ongoing clinical trials, the biotech would only be able to finance itself through October before having to wind down operations.
Despite having to help fund ixo-vec’s development, which has been granted fast track and regenerative medicine advanced therapy (RMAT) designations by the US Food and Drug Administration (FDA), Eli Lilly could use the candidate to enter the lucrative wAMD market. The AMD sector, which also includes the dry form, is expected to reach $27.5bn across 7MM by 2031 (7MM: US, France, Germany, Italy, Spain, UK, and Japan), according to GlobalData analysis.
There is no gene therapy approved with a wAMD indication, with current treatments working via the anti–vascular endothelial growth factor (VEGF) mechanism, such as Regeneron’s blockbuster Eylea (aflibercept). The therapy is administered every four weeks for the first five months, followed by a single injection every two months. For Eli Lilly’s soon-to-be acquired ixo-vec, this could offer patients a one-and-done treatment.
Lilly molecule discovery group vice-president Andrew Adams said: “Ixo-vec has the potential to transform wAMD treatment from a paradigm of chronic care with repeated intravitreal injections to a convenient one-time therapy.”
Adverum CEO Laurent Fischer: “[Lilly’s] scientific depth and global reach offer the opportunity to accelerate our vision to deliver a transformative one-and-done therapy that can potentially restore and preserve vision for millions of patients living with wAMD.”
Lilly bucks big pharma trend
This is not the first time in 2025 that Eli Lilly has swooped in to rescue a cash-strapped biotech specialising in gene therapies. In April, the big pharma signed a licensing deal worth up to $1.4bn for Sangamo Therapeutics’ neurology-targeting gene therapy.
However, Lilly’s recent deals, which includes a $1.3bn acquisition of RNA-based gene therapy developer Rznomics in May 2025, goes against the grain of big pharma generally opting to retreat from the cell and gene therapy sector.
Earlier this month, Galapagos wound down its cell and gene therapy division after failing to sell the unit. Japanese pharma Takeda also abandoned its cell therapy research, pivoting instead towards small molecules, biologics and antibody-drug conjugates (ADCs).
In addition, Gilead Sciences’ Kite Pharma terminated its cell therapy collaboration with Shoreline in September 2025, ending a research partnership valued at $2.3bn.
Cell & Gene Therapy coverage on Pharmaceutical Technology is supported by Cytiva.
Editorial content is independently produced and follows the highest standards of journalistic integrity. Topic sponsors are not involved in the creation of editorial content.
Continue Reading


