This request seems a bit unusual, so we need to confirm that you’re human. Please press and hold the button until it turns completely green. Thank you for your cooperation!
Blog
-
Just a moment…
Just a moment… -

Melting ice protects Antarctica’s ocean against carbon disaster: Study
A new study challenges previous climate predictions by demonstrating that climate change has impacted the Southern Ocean’s ability to absorb carbon dioxide. However, a delicate relationship remains in the balance.
According to scientists…
Continue Reading
-
Medvedev ends 882-day title drought in Almaty – ATP Tour
- Medvedev ends 882-day title drought in Almaty ATP Tour
- Medvedev breaks semifinal curse to reach Almaty final Mundo Deportivo
- ATP Almaty James Duckworth vs Daniil Medvedev 🧠 Form & Context 🇦🇺 James Duckworth (#138, righty, 183 cm) 2025:…
Continue Reading
-

ALEX Trial Final Results at ESMO 2025: Alectinib Demonstrates Durable Overall Survival and Long-Term Disease Control in Advanced ALK-Positive NSCLC
The ALEX trial (NCT02075840) represents one of the most pivotal studies in the evolution of treatment for anaplastic lymphoma kinase–positive (ALK+) non–small cell lung cancer (NSCLC). The trial’s earlier findings led to the global approval of alectinib as the first-line standard of care, demonstrating significant improvements in progression-free survival (PFS) and central nervous system (CNS) disease control compared with crizotinib.
At the ESMO Congress 2025, investigators presented the final overall survival (OS) and duration of response (DoR) results from ALEX, alongside updated long-term safety findings. These mature data confirm the sustained clinical benefit of first-line alectinib over crizotinib, with remarkable long-term survival extending beyond 80 months in the overall population and consistent benefit across all CNS subgroups.
Methods
The ALEX study was a randomized, phase 3, open-label trial enrolling 303 patients aged ≥18 years with previously untreated, advanced ALK+ NSCLC. Participants were randomized 1:1 to receive either:
- Alectinib 600 mg twice daily (BID), or
- Crizotinib 250 mg BID,
administered until disease progression, unacceptable toxicity, withdrawal, or death.
Randomization was stratified by ECOG performance status (0–1 vs 2), race (Asian vs non-Asian), and baseline CNS metastases (yes vs no). Notably, crossover between treatment arms before disease progression was not permitted, ensuring the validity of survival comparisons.
The key secondary endpoints included overall survival (OS), duration of response (DoR), and safety.
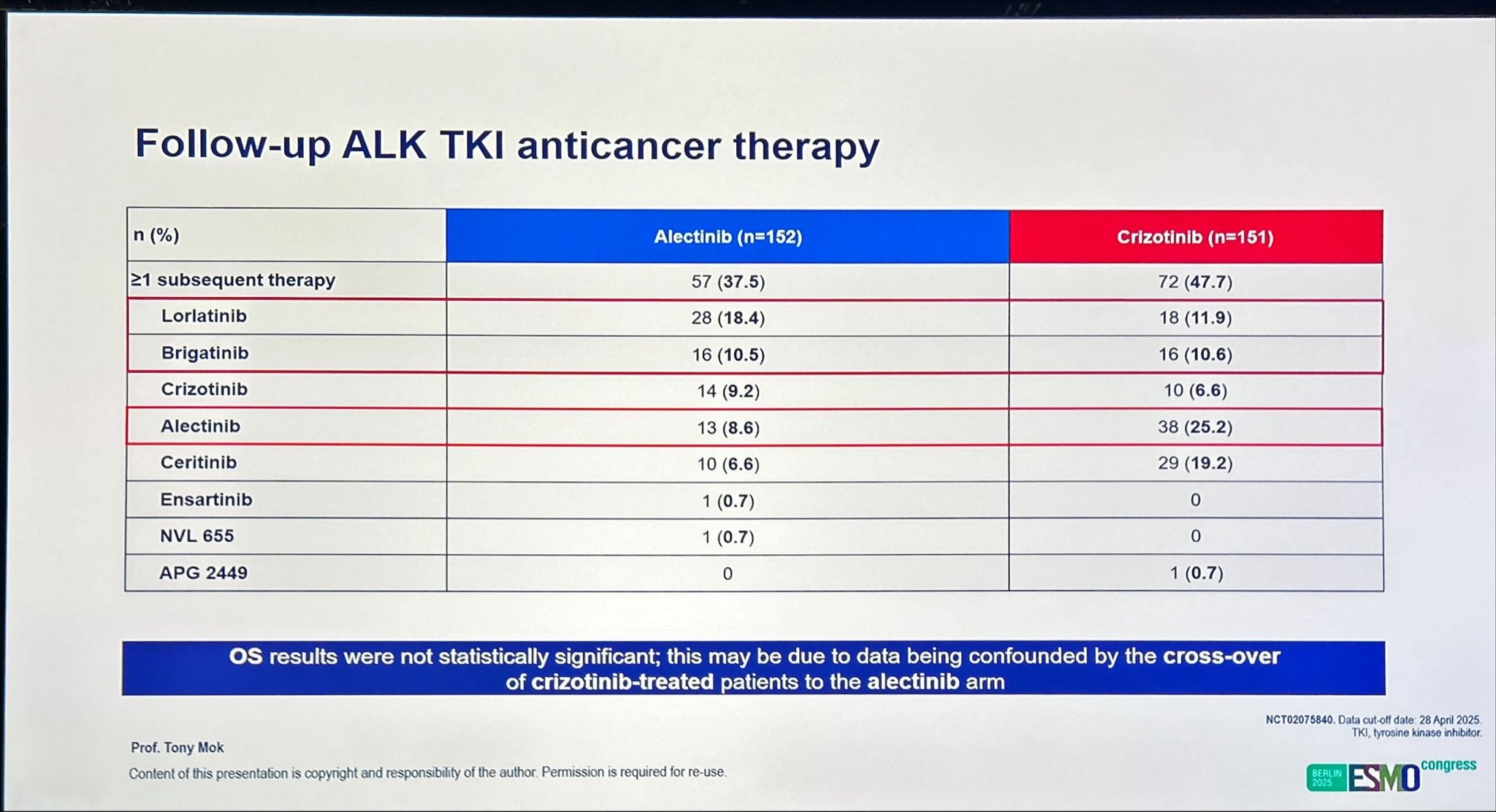
Results
At the final data cutoff of April 28, 2025, a total of 152 patients had been assigned to alectinib and 151 to crizotinib. After a median follow-up of 53.5 months for alectinib and 23.3 months for crizotinib, the median OS was significantly prolonged with alectinib, reaching 81.1 months (95% CI, 62.3–not estimable) compared with 54.2 months (95% CI, 34.6–75.6) for crizotinib. This corresponds to a hazard ratio (HR) of 0.78 (95% CI, 0.56–1.08), reflecting a 22% reduction in the risk of death.
Extended Overall Survival and Crossover Considerations
At a median follow-up of 53.5 months for the alectinib arm and 23.3 months for the crizotinib arm, the 7-year overall survival (OS) rate reached 48.6 % with alectinib compared with 38.2 % with crizotinib, confirming a durable long-term benefit. The OS hazard ratio (HR 0.78; 95 % CI 0.56–1.08) favored alectinib, reflecting a clinically meaningful 22 % reduction in the risk of death. While the difference did not reach formal statistical significance, this is likely due to crossover of crizotinib-treated patients to subsequent ALK TKI therapy, a factor known to confound late-phase survival outcomes. These findings underscore the robustness of alectinib’s efficacy, even in the context of real-world treatment sequencing.
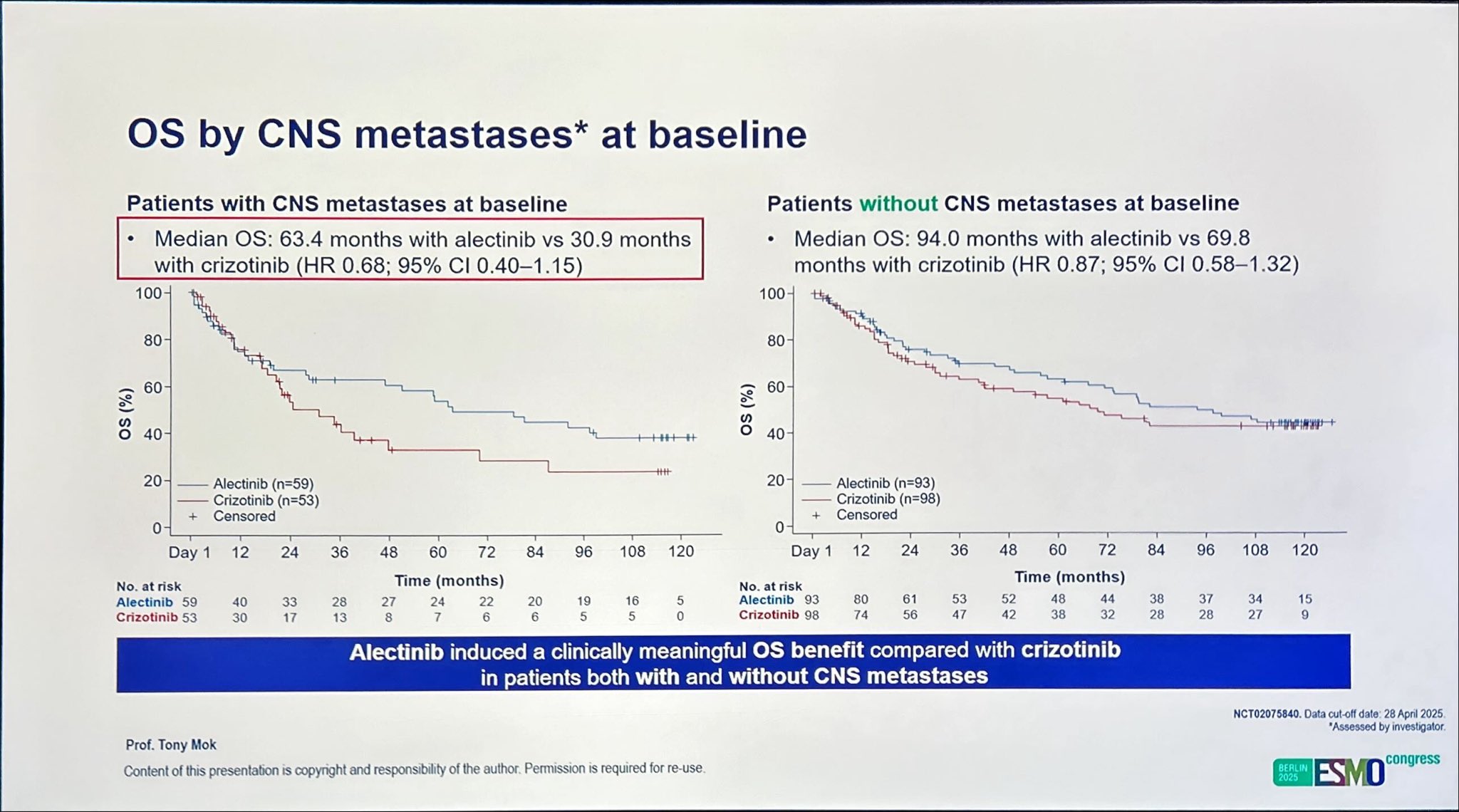
CNS Subgroup Analysis
A particularly compelling aspect of the ALEX trial is the long-term CNS efficacy of alectinib, a known CNS-penetrant ALK inhibitor.
- Among patients with baseline CNS metastases and prior radiation, median OS was 92.0 months with alectinib vs 39.5 months with crizotinib.
- In those with CNS metastases but no prior radiation, median OS was 46.9 months vs 23.7 months, respectively.
- In patients without baseline CNS metastases, median OS reached 94.0 months vs 69.8 months, underscoring the durability of systemic and intracranial control with alectinib.
Duration of Response
The median duration of response (DoR) was 42.3 months (95% CI, 31.3–51.3) with alectinib compared to only 11.1 months (95% CI, 7.9–13.0) with crizotinib (HR 0.41; 95% CI, 0.30–0.56).**
This nearly fourfold improvement in DoR illustrates the sustained and deep responses achieved with alectinib, consistent with its superior CNS efficacy and tolerability profile.
Safety Profile
Alectinib demonstrated a favorable and manageable safety profile, consistent with prior analyses and its established global experience.
- Median treatment duration was 28.1 months with alectinib versus 10.8 months with crizotinib, reflecting prolonged disease control.
- Grade 3–5 adverse events (AEs) occurred in 57.9% (alectinib) and 57.6% (crizotinib) of patients.
- Serious AEs were reported in 46.1% (alectinib) and 31.8% (crizotinib).
- Treatment discontinuation due to AEs occurred in 17.8% vs 14.6%, respectively.
Importantly, no new or unexpected safety concerns emerged, affirming the long-term tolerability of alectinib in prolonged use.
Interpretation
The final analysis from the ALEX trial confirms that first-line alectinib delivers durable, clinically meaningful survival benefit over crizotinib, with median overall survival surpassing 6.5 years. These results are particularly striking given that crossover was not allowed before disease progression, ensuring that the OS benefit reflects true treatment effect rather than post-progression therapy influence.
Furthermore, the robust CNS efficacy of alectinib remains a defining feature, addressing one of the key unmet needs in ALK+ NSCLC—prevention and long-term control of brain metastases.
Patients with CNS involvement achieved median OS approaching 8 years when treated with alectinib, compared to just over 3 years with crizotinib, underscoring the clinical importance of CNS-active targeted therapy.
Long-Term Follow-Up and Analytical Considerations
The ALEX final OS analysis at ESMO 2025 provides the most mature survival data for any first-line ALK inhibitor to date. With a median follow-up exceeding four years, the survival curves for alectinib versus crizotinib remain distinctly separated, showing no late convergence, which supports the long-lasting disease control achieved with alectinib.
These results also emphasize the reliability of long-term ALK inhibition and suggest that continuous blockade of ALK signaling may delay the emergence of resistance mechanisms that typically limit the efficacy of earlier-generation TKIs.
From a methodological standpoint, the no-crossover design strengthens the validity of the OS findings by minimizing confounding from post-progression therapy. The consistent DoR benefit further supports the durable and deep responses achievable with alectinib.
The trial’s safety monitoring over more than five years confirms the absence of cumulative toxicity, reaffirming alectinib’s suitability for prolonged therapy. These findings collectively reinforce alectinib as the definitive first-line standard of care for patients with advanced ALK-positive NSCLC.
Read Full Abstract on ESMO Congress 2025 Website
Written by Armen Gevorgyan, MD
Continue Reading
-
Rune provides injury update – ATP Tour
- Rune provides injury update ATP Tour
- ‘Sport must adapt’, says Draper after Rune injury BBC
- Jack Draper demands ATP Tour action after Novak Djokovic retires and Taylor Fritz agrees Daily Express
- Holger Rune suffers probable Achilles injury at…
Continue Reading
-

A Director of Engineering Shares His Career Path and Management Insights
Managing engineering teams has always required a careful mix of technical depth and people skills. But as artificial intelligence reshapes how code is created, that equation is changing. Leaders now face new questions. How can they integrate AI…
Continue Reading
-

DP World Tour: Tommy Fleetwood wins in India to answer son’s dreams
Fleetwood had started the final round in second place, two shots behind Japan’s Keita Nakajima.
New Zealander Daniel Hillier – ranked 218th in the world – leapfrogged them into a surprise lead, after moving to seven under for his round, but was…
Continue Reading
-

Dato-DXd Doubles Response Rates and Extends Survival in First-Line Metastatic TNBC
Q: Could you summarize the magnitude of benefit seen with Dato-DXd compared to chemotherapy in this first-line mTNBC population and comment on how clinically meaningful this is for practice?
Rebecca Dent, MD: As we know, patients with metastatic triple-negative breast cancer—especially those who are not eligible for immune checkpoint inhibition, which represents about 60% to 70% of patients we see in the first-line setting—have had chemotherapy as the standard of care for well over 10 years. In this setting, 50% of patients progress and never go on to receive second-line therapy. So there was a real need to incorporate a novel therapy into the first-line setting.In addition, patients are receiving more treatment in the curative setting, and so if they relapse, they’ve already seen a number of different chemotherapies. What’s unique about TROPION-Breast02 is that we are incorporating an antibody-drug conjugate as the first-line therapy in patients with relapsed or de novo metastatic TNBC, or those relapsing more than 12 months after initial therapy.
Here we saw the use of Dato-DXd, a TROP2-directed antibody-drug conjugate with a potent topoisomerase payload and a stable, cleavable linker. Because of the drug-to-antibody ratio, we see potent effectiveness in terms of improvements in progression-free survival (PFS), with a safety profile that allows administration every three weeks, which is very convenient for patients.
We saw an impressive improvement in PFS—from about 5 months to over 10 months in median PFS, a delta of more than 5 months. These Kaplan-Meier curves separate very early, with a time to treatment response of 1.4 months, and the curves continue to separate and remain sustained.
We also saw an improvement in overall survival (OS). Median OS was 18.7 months with standard chemotherapy and increased to almost 2 years with Dato-DXd—a delta of 5 months, with a statistically significant hazard ratio.
The data were very consistent. In clinic, what’s extraordinary is seeing such a visible improvement in response. We saw more than a doubling of response rates with Dato-DXd compared with chemotherapy—well over 60%, a delta of more than 30%, and a tripling of the complete response rate.
What’s even more helpful in clinic is that these responses are sustained. Often, we can achieve a response, but it doesn’t last. In this trial, duration of response increased from 7 months with chemotherapy to more than 12 months with Dato-DXd—again, a 5-month improvement. So across endpoints—PFS, OS, and duration of response—we consistently saw about a 5-month delta favoring Dato-DXd.
Continue Reading
-

AAO 2025: Which lens to consider in patients with pseudoexfoliation | Ophthalmology Times
Note: Video captions are generated with the assistance of AI and may contain errors.
John P. Berdahl, MD, a surgeon at Vance Thompson Vision and a professor at the University of South Dakota, spoke on the lens implantation strategies for patients…
Continue Reading
