Wazir Mohammad, Pakistan’s oldest living cricket player, died at 95 in Birmingham, United Kingdom on Monday.He was the eldest among the famous Mohammad brothers and played 20 Test matches for Pakistan between 1952…
Blog
-

Winning this event never feels routine
Since claiming his first title in 2015, Omar Assar has become the most decorated table tennis player in the history of the ITTF-Africa Championships, having lifted the trophy three more times (2016, 2021, 2024).
Despite these impressive…
Continue Reading
-
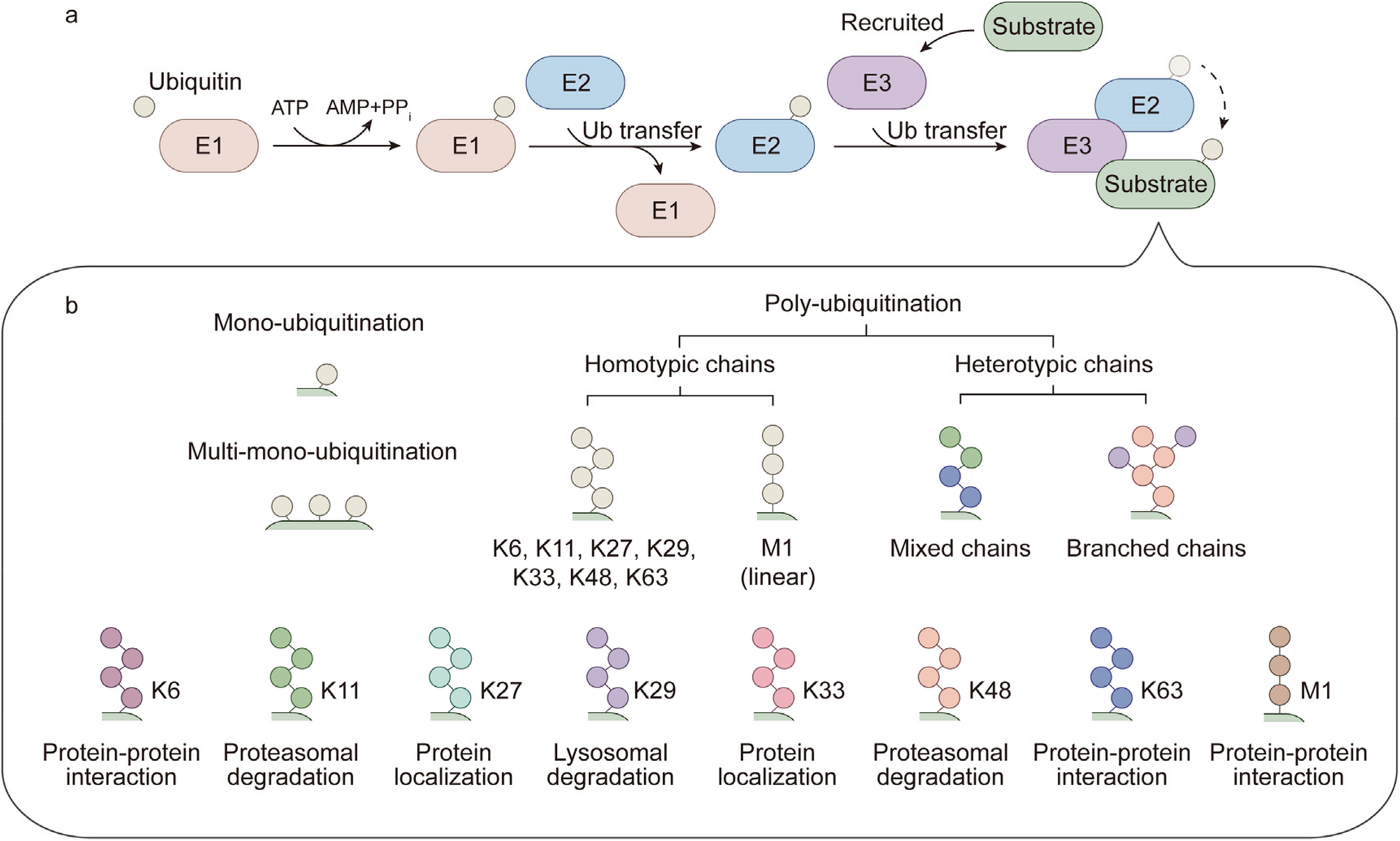
F-box proteins in cancer: from cancer cells to the tumor microenvironment | Cell Communication and Signaling
Swatek KN, Komander D. Ubiquitin modifications. Cell Res. 2016;26(4):399–422.
Google Scholar
Pickart CM. Mechanisms underlying ubiquitination. Annu Rev Biochem. 2001;70:503–33.
Google Scholar
Hershko A, Ciechanover A. The ubiquitin system. Annu Rev Biochem. 1998;67:425–79.
Google Scholar
Dikic I, Schulman BA. An expanded lexicon for the ubiquitin code. Nat Rev Mol Cell Biol. 2023;24(4):273–87.
Google Scholar
Chen ZJ, Sun LJ. Nonproteolytic functions of ubiquitin in cell signaling. Mol Cell. 2009;33(3):275–86.
Google Scholar
Kulathu Y, Komander D. Atypical ubiquitylation – the unexplored world of Polyubiquitin beyond Lys48 and Lys63 linkages. Nat Rev Mol Cell Biol. 2012;13(8):508–23.
Google Scholar
Yuan WC, Lee YR, Lin SY, Chang LY, Tan YP, Hung CC, et al. K33-Linked polyubiquitination of Coronin 7 by Cul3-KLHL20 ubiquitin E3 ligase regulates protein trafficking. Mol Cell. 2014;54(4):586–600.
Google Scholar
Ulrich HD, Walden H. Ubiquitin signalling in DNA replication and repair. Nat Rev Mol Cell Biol. 2010;11(7):479–89.
Google Scholar
Liu F, Chen J, Li K, Li H, Zhu Y, Zhai Y, et al. Ubiquitination and deubiquitination in cancer: from mechanisms to novel therapeutic approaches. Mol Cancer. 2024;23(1):148.
Google Scholar
Morreale FE, Walden H. Types of ubiquitin ligases. Cell. 2016;165(1):248–e1.
Google Scholar
Petroski MD, Deshaies RJ. Function and regulation of cullin-RING ubiquitin ligases. Nat Rev Mol Cell Biol. 2005;6(1):9–20.
Google Scholar
Skaar JR, Florens L, Tsutsumi T, Arai T, Tron A, Swanson SK, et al. PARC and CUL7 form atypical Cullin RING ligase complexes. Cancer Res. 2007;67(5):2006–14.
Google Scholar
Baek K, Scott DC, Henneberg LT, King MT, Mann M, Schulman BA. Systemwide disassembly and assembly of SCF ubiquitin ligase complexes. Cell. 2023;186(9):1895–911. e21.
Google Scholar
Reitsma JM, Liu X, Reichermeier KM, Moradian A, Sweredoski MJ, Hess S, et al. Composition and regulation of the cellular repertoire of SCF ubiquitin ligases. Cell. 2017;171(6):1326–e3914.
Google Scholar
Jin J, Cardozo T, Lovering RC, Elledge SJ, Pagano M, Harper JW. Systematic analysis and nomenclature of mammalian F-box proteins. Genes Dev. 2004;18(21):2573–80.
Google Scholar
Liu Y, Jurczak MJ, Lear TB, Lin B, Larsen MB, Kennerdell JR, et al. A Fbxo48 inhibitor prevents pAMPKalpha degradation and ameliorates insulin resistance. Nat Chem Biol. 2021;17(3):298–306.
Google Scholar
Simon-Kayser B, Scoul C, Renaudin K, Jezequel P, Bouchot O, Rigaud J, et al. Molecular cloning and characterization of FBXO47, a novel gene containing an F-box domain, located in the 17q12 band deleted in papillary renal cell carcinoma. Genes Chromosomes Cancer. 2005;43(1):83–94.
Google Scholar
Hu J, Zacharek S, He YJ, Lee H, Shumway S, Duronio RJ, et al. WD40 protein FBW5 promotes ubiquitination of tumor suppressor TSC2 by DDB1-CUL4-ROC1 ligase. Genes Dev. 2008;22(7):866–71.
Google Scholar
Hopf LVM, Baek K, Klugel M, von Gronau S, Xiong Y, Schulman BA. Structure of CRL7(FBXW8) reveals coupling with CUL1-RBX1/ROC1 for multi-cullin-RING E3-catalyzed ubiquitin ligation. Nat Struct Mol Biol. 2022;29(9):854–62.
Google Scholar
Zheng N, Shabek N. Ubiquitin ligases: Structure, Function, and regulation. Annu Rev Biochem. 2017;86:129–57.
Google Scholar
Frescas D, Pagano M. Deregulated proteolysis by the F-box proteins SKP2 and beta-TrCP: tipping the scales of cancer. Nat Rev Cancer. 2008;8(6):438–49.
Google Scholar
Orlicky S, Tang X, Willems A, Tyers M, Sicheri F. Structural basis for phosphodependent substrate selection and orientation by the SCFCdc4 ubiquitin ligase. Cell. 2003;112(2):243–56.
Google Scholar
Abbas T, Mueller AC, Shibata E, Keaton M, Rossi M, Dutta A. CRL1-FBXO11 promotes Cdt2 ubiquitylation and degradation and regulates Pr-Set7/Set8-mediated cellular migration. Mol Cell. 2013;49(6):1147–58.
Google Scholar
Kuchay S, Duan S, Schenkein E, Peschiaroli A, Saraf A, Florens L, et al. FBXL2- and PTPL1-mediated degradation of p110-free p85beta regulatory subunit controls the PI(3)K signalling cascade. Nat Cell Biol. 2013;15(5):472–80.
Google Scholar
Skaar JR, Pagan JK, Pagano M. Mechanisms and function of substrate recruitment by F-box proteins. Nat Rev Mol Cell Biol. 2013;14(6):369–81.
Google Scholar
Yoshida Y, Chiba T, Tokunaga F, Kawasaki H, Iwai K, Suzuki T, et al. E3 ubiquitin ligase that recognizes sugar chains. Nature. 2002;418(6896):438–42.
Google Scholar
Yoshida Y, Tokunaga F, Chiba T, Iwai K, Tanaka K, Tai T. Fbs2 is a new member of the E3 ubiquitin ligase family that recognizes sugar chains. J Biol Chem. 2003;278(44):43877–84.
Google Scholar
D’Angiolella V, Donato V, Forrester FM, Jeong YT, Pellacani C, Kudo Y, et al. Cyclin F-mediated degradation of ribonucleotide reductase M2 controls genome integrity and DNA repair. Cell. 2012;149(5):1023–34.
Google Scholar
D’Angiolella V, Donato V, Vijayakumar S, Saraf A, Florens L, Washburn MP, et al. SCF(Cyclin F) controls centrosome homeostasis and mitotic fidelity through CP110 degradation. Nature. 2010;466(7302):138–42.
Google Scholar
Cao S, Garcia SF, Shi H, James EI, Kito Y, Shi H, et al. Recognition of BACH1 quaternary structure degrons by two F-box proteins under oxidative stress. Cell. 2024;187(26):7568–e8422.
Google Scholar
Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100(1):57–70.
Google Scholar
Randle SJ, Laman H. F-box protein interactions with the hallmark pathways in cancer. Semin Cancer Biol. 2016;36:3–17.
Google Scholar
Wang Z, Liu P, Inuzuka H, Wei W. Roles of F-box proteins in cancer. Nat Rev Cancer. 2014;14(4):233–47.
Google Scholar
Xu J, Zhou W, Yang F, Chen G, Li H, Zhao Y, et al. The beta-TrCP-FBXW2-SKP2 axis regulates lung cancer cell growth with FBXW2 acting as a tumour suppressor. Nat Commun. 2017;8:14002.
Google Scholar
Islam S, Dutta P, Sahay O, Gopalakrishnan K, Muhury SR, Parameshwar P, et al. Feedback-regulated transcriptional repression of FBXO31 by c-Myc triggers ovarian cancer tumorigenesis. Int J Cancer. 2022;150(9):1512–24.
Google Scholar
Hanahan D. Hallmarks of cancer: new dimensions. Cancer Discov. 2022;12(1):31–46.
Google Scholar
Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144(5):646–74.
Google Scholar
Perez-Gonzalez A, Bevant K, Blanpain C. Cancer cell plasticity during tumor progression, metastasis and response to therapy. Nat Cancer. 2023;4(8):1063–82.
Google Scholar
Nieto MA, Huang RY, Jackson RA, Thiery JP. Emt: 2016 Cell. 2016;166(1):21–45.
Google Scholar
Le Magnen C, Shen MM, Abate-Shen C. Lineage plasticity in cancer progression and treatment. Annu Rev Cancer Biol. 2018;2:271–89.
Google Scholar
Sun R, Xie HY, Qian JX, Huang YN, Yang F, Zhang FL, et al. FBXO22 possesses both protumorigenic and antimetastatic roles in breast cancer progression. Cancer Res. 2018;78(18):5274–86.
Google Scholar
Zou S, Ma C, Yang F, Xu X, Jia J, Liu Z. FBXO31 suppresses gastric cancer EMT by targeting Snail1 for proteasomal degradation. Mol Cancer Res. 2018;16(2):286–95.
Google Scholar
Ryu KJ, Park SM, Park SH, Kim IK, Han H, Kim HJ, et al. p38 stabilizes snail by suppressing DYRK2-Mediated phosphorylation that is required for GSK3beta-betaTrCP-Induced snail degradation. Cancer Res. 2019;79(16):4135–48.
Google Scholar
Zhang Y, Zhang X, Ye M, Jing P, Xiong J, Han Z, et al. FBW7 loss promotes epithelial-to-mesenchymal transition in non-small cell lung cancer through the stabilization of snail protein. Cancer Lett. 2018;419:75–83.
Google Scholar
Cuevas IC, Sahoo SS, Kumar A, Zhang H, Westcott J, Aguilar M, et al. Fbxw7 is a driver of uterine carcinosarcoma by promoting epithelial-mesenchymal transition. Proc Natl Acad Sci U S A. 2019;116(51):25880–90.
Google Scholar
Qiao X, Lin J, Shen J, Chen Y, Zheng L, Ren H, et al. FBXO28 suppresses liver cancer invasion and metastasis by promoting PKA-dependent SNAI2 degradation. Oncogene. 2023;42(39):2878–91.
Google Scholar
Kim HY, Kim YM, Hong S. DNAJB9 suppresses the metastasis of triple-negative breast cancer by promoting FBXO45-mediated degradation of ZEB1. Cell Death Dis. 2021;12(5):461.
Google Scholar
Cui YH, Kang JH, Suh Y, Zhao Y, Yi JM, Bae IH, et al. Loss of FBXL14 promotes mesenchymal shift and radioresistance of non-small cell lung cancer by TWIST1 stabilization. Signal Transduct Target Ther. 2021;6(1):272.
Google Scholar
Mo Y, Wang Y, Wang Y, Deng X, Yan Q, Fan C, et al. Circular RNA circPVT1 promotes nasopharyngeal carcinoma metastasis via the beta-TrCP/c-Myc/SRSF1 positive feedback loop. Mol Cancer. 2022;21(1):192.
Google Scholar
Podmirseg SR, Jakel H, Ranches GD, Kullmann MK, Sohm B, Villunger A, et al. Caspases uncouple p27(Kip1) from cell cycle regulated degradation and abolish its ability to stimulate cell migration and invasion. Oncogene. 2016;35(35):4580–90.
Google Scholar
Sahu SK, Tiwari N, Pataskar A, Zhuang Y, Borisova M, Diken M, et al. FBXO32 promotes microenvironment underlying epithelial-mesenchymal transition via CtBP1 during tumour metastasis and brain development. Nat Commun. 2017;8(1):1523.
Google Scholar
Bagger SO, Hopkinson BM, Pandey DP, Bak M, Brydholm AV, Villadsen R, et al. Aggressiveness of non-EMT breast cancer cells relies on FBXO11 activity. Mol Cancer. 2018;17(1):171.
Google Scholar
Zhao Y, Shim N, Cui YH, Kang JH, Yoo KC, Kim S, et al. FBXO15 plays a critical suppressive functional role in regulation of breast cancer progression. Signal Transduct Target Ther. 2021;6(1):211.
Google Scholar
Cao J, Zhao M, Liu J, Zhang X, Pei Y, Wang J, et al. RACK1 promotes Self-Renewal and chemoresistance of cancer stem cells in human hepatocellular carcinoma through stabilizing Nanog. Theranostics. 2019;9(3):811–28.
Google Scholar
Xiao G, Lu W, Yuan J, Liu Z, Wang P, Fan H. Fbxw7 suppresses carcinogenesis and stemness in triple-negative breast cancer through CHD4 degradation and Wnt/beta-catenin pathway Inhibition. J Transl Med. 2024;22(1):99.
Google Scholar
Yin Y, Xie CM, Li H, Tan M, Chen G, Schiff R, et al. The FBXW2-MSX2-SOX2 axis regulates stem cell property and drug resistance of cancer cells. Proc Natl Acad Sci U S A. 2019;116(41):20528–38.
Google Scholar
Zhao X, Shu D, Sun W, Si S, Ran W, Guo B, et al. PLEK2 promotes cancer stemness and tumorigenesis of head and neck squamous cell carcinoma via the c-Myc-mediated positive feedback loop. Cancer Commun (Lond). 2022;42(10):987–1007.
Google Scholar
Yao J, Wang XP, Yang J, Yang Z, Zhang ZY. SCF-FBXL8 contributes to liver metastasis and stem-cell-like features in colorectal cancer cells by mediating ubiquitination and degradation of TP53. Clin Transl Med. 2023;13(3):e1208.
Google Scholar
Zhu X, Wang F, Wu X, Li Z, Wang Z, Ren X, et al. FBX8 promotes metastatic dormancy of colorectal cancer in liver. Cell Death Dis. 2020;11(8):622.
Google Scholar
Fontana R, Mestre-Farrera A, Yang J. Update on Epithelial-Mesenchymal plasticity in cancer progression. Annu Rev Pathol. 2024;19:133–56.
Google Scholar
Banito A, Li X, Laporte AN, Roe JS, Sanchez-Vega F, Huang CH, et al. The SS18-SSX oncoprotein hijacks KDM2B-PRC1.1 to drive synovial sarcoma. Cancer Cell. 2018;33(3):527–41. e8.
Google Scholar
Li S, Chen Y, Xie Y, Zhan H, Zeng Y, Zeng K, et al. FBXO7 confers mesenchymal properties and chemoresistance in glioblastoma by controlling Rbfox2-Mediated alternative splicing. Adv Sci (Weinh). 2023;10(33):e2303561.
Google Scholar
Debnath J, Gammoh N, Ryan KM. Autophagy and autophagy-related pathways in cancer. Nat Rev Mol Cell Biol. 2023;24(8):560–75.
Google Scholar
Zhao Y, Xiong X, Sun Y. DEPTOR, an mTOR inhibitor, is a physiological substrate of SCF(betaTrCP) E3 ubiquitin ligase and regulates survival and autophagy. Mol Cell. 2011;44(2):304–16.
Google Scholar
Xie CM, Tan M, Lin XT, Wu D, Jiang Y, Tan Y, et al. The FBXW7-SHOC2-Raptor axis controls the Cross-Talks between the RAS-ERK and mTORC1 signaling pathways. Cell Rep. 2019;26(11):3037–50. e4.
Google Scholar
Suzuki N, Johmura Y, Wang TW, Migita T, Wu W, Noguchi R, et al. TP53/p53-FBXO22-TFEB controls basal autophagy to govern hormesis. Autophagy. 2021;17(11):3776–93.
Google Scholar
Zhang Z, Liu X, Chu C, Zhang Y, Li W, Yu X, et al. MIR937 amplification potentiates ovarian cancer progression by attenuating FBXO16 Inhibition on ULK1-mediated autophagy. Cell Death Dis. 2024;15(10):735.
Google Scholar
Song W, Zeng Z, Zhang Y, Li H, Cheng H, Wang J, et al. CircRNF144B/miR-342-3p/FBXL11 axis reduced autophagy and promoted the progression of ovarian cancer by increasing the ubiquitination of Beclin-1. Cell Death Dis. 2022;13(10):857.
Google Scholar
Vargas JNS, Hamasaki M, Kawabata T, Youle RJ, Yoshimori T. The mechanisms and roles of selective autophagy in mammals. Nat Rev Mol Cell Biol. 2023;24(3):167–85.
Google Scholar
Deng R, Zhang HL, Huang JH, Cai RZ, Wang Y, Chen YH, et al. MAPK1/3 kinase-dependent ULK1 degradation attenuates mitophagy and promotes breast cancer bone metastasis. Autophagy. 2021;17(10):3011–29.
Google Scholar
Nguyen-Dien GT, Townsend B, Kulkarni PG, Kozul KL, Ooi SS, Eldershaw DN, et al. PPTC7 antagonizes mitophagy by promoting BNIP3 and NIX degradation via SCF(FBXL4). EMBO Rep. 2024;25(8):3324–47.
Google Scholar
Yoshida Y, Yasuda S, Fujita T, Hamasaki M, Murakami A, Kawawaki J, et al. Ubiquitination of exposed glycoproteins by SCF(FBXO27) directs damaged lysosomes for autophagy. Proc Natl Acad Sci U S A. 2017;114(32):8574–9.
Google Scholar
Cui D, Dai X, Shu J, Ma Y, Wei D, Xiong X, et al. The cross talk of two family members of beta-TrCP in the regulation of cell autophagy and growth. Cell Death Differ. 2020;27(3):1119–33.
Google Scholar
Chen X, Kang R, Kroemer G, Tang D. Broadening horizons: the role of ferroptosis in cancer. Nat Rev Clin Oncol. 2021;18(5):280–96.
Google Scholar
Huang G, Xiang Z, Wu H, He Q, Dou R, Lin Z, et al. The LncRNA BDNF-AS/WDR5/FBXW7 axis mediates ferroptosis in gastric cancer peritoneal metastasis by regulating VDAC3 ubiquitination. Int J Biol Sci. 2022;18(4):1415–33.
Google Scholar
Ye Z, Zhuo Q, Hu Q, Xu X, Mengqi L, Zhang Z, et al. FBW7-NRA41-SCD1 axis synchronously regulates apoptosis and ferroptosis in pancreatic cancer cells. Redox Biol. 2021;38:101807.
Google Scholar
Chen TC, Chuang JY, Ko CY, Kao TJ, Yang PY, Yu CH, et al. AR ubiquitination induced by the Curcumin analog suppresses growth of temozolomide-resistant glioblastoma through disrupting GPX4-Mediated redox homeostasis. Redox Biol. 2020;30:101413.
Google Scholar
Zhu Z, Zheng Y, He H, Yang L, Yang J, Li M, et al. FBXO31 sensitizes cancer stem cells-like cells to cisplatin by promoting ferroptosis and facilitating proteasomal degradation of GPX4 in cholangiocarcinoma. Liver Int. 2022;42(12):2871–88.
Google Scholar
Zhou P, Peng X, Zhang K, Cheng J, Tang M, Shen L, et al. HAT1/HDAC2 mediated ACSL4 acetylation confers radiosensitivity by inducing ferroptosis in nasopharyngeal carcinoma. Cell Death Dis. 2025;16(1):160.
Google Scholar
Zhou Q, Meng Y, Li D, Yao L, Le J, Liu Y, et al. Ferroptosis in cancer: from molecular mechanisms to therapeutic strategies. Signal Transduct Target Ther. 2024;9(1):55.
Google Scholar
Lei G, Zhuang L, Gan B. The roles of ferroptosis in cancer: tumor suppression, tumor microenvironment, and therapeutic interventions. Cancer Cell. 2024;42(4):513–34.
Google Scholar
Denton D, Kumar S. Autophagy-dependent cell death. Cell Death Differ. 2019;26(4):605–16.
Google Scholar
Liang Y, Chen P, Wang S, Cai L, Zhu F, Jiang Y, et al. SCF(FBXW5)-mediated degradation of AQP3 suppresses autophagic cell death through the PDPK1-AKT-MTOR axis in hepatocellular carcinoma cells. Autophagy. 2024;20(9):1984–99.
Google Scholar
Agrawal Y, Sharma T, Islam S, Nadkarni KS, Santra MK. F-box protein FBXO41 suppresses breast cancer growth by inducing autophagic cell death through facilitating proteasomal degradation of oncogene SKP2. Int J Biochem Cell Biol. 2022;147:106228.
Google Scholar
Li M, Lu H, Ruan C, Ke Q, Hu L, Li Z, et al. CircMAPK1 induces cell pyroptosis in sepsis-induced lung injury by mediating KDM2B mRNA decay to epigenetically regulate WNK1. Mol Med. 2024;30(1):155.
Google Scholar
Wang Q, Huang PY, Wu JG, Zhang TQ, Li LF, Huang LD, et al. miR-219a-5p inhibits the pyroptosis in knee osteoarthritis by inactivating the NLRP3 signaling via targeting FBXO3. Environ Toxicol. 2022;37(11):2673–82.
Google Scholar
Faubert B, Solmonson A, DeBerardinis RJ. Metabolic reprogramming and cancer progression. Science. 2020;368(6487):eaaw5473.
Hu T, Liu H, Liang Z, Wang F, Zhou C, Zheng X, et al. Tumor-intrinsic CD47 signal regulates Glycolysis and promotes colorectal cancer cell growth and metastasis. Theranostics. 2020;10(9):4056–72.
Google Scholar
Zhang P, Shao Y, Quan F, Liu L, Yang J. FBP1 enhances the radiosensitivity by suppressing Glycolysis via the FBXW7/mTOR axis in nasopharyngeal carcinoma cells. Life Sci. 2021;283:119840.
Google Scholar
Ji S, Qin Y, Liang C, Huang R, Shi S, Liu J, et al. FBW7 (F-box and WD repeat Domain-Containing 7) negatively regulates glucose metabolism by targeting the c-Myc/TXNIP (Thioredoxin-Binding Protein) axis in pancreatic cancer. Clin Cancer Res. 2016;22(15):3950–60.
Google Scholar
Guo X, Zhu Y, Hong X, Zhang M, Qiu X, Wang Z, et al. miR-181d and c-myc-mediated Inhibition of CRY2 and FBXL3 reprograms metabolism in colorectal cancer. Cell Death Dis. 2017;8(7):e2958.
Google Scholar
Chan CH, Li CF, Yang WL, Gao Y, Lee SW, Feng Z, et al. The Skp2-SCF E3 ligase regulates Akt ubiquitination, glycolysis, Herceptin sensitivity, and tumorigenesis. Cell. 2012;149(5):1098–111.
Google Scholar
Rong Z, Yang J, Liu J, Meng Q, Hua J, Tan Z, et al. Dense stroma activates the TGF-beta1/FBW7 axis to induce metabolic subtype switching in pancreatic cancer. Int J Surg. 2025;111(2):1891–903.
Google Scholar
Wei W, Qin B, Wen W, Zhang B, Luo H, Wang Y, et al. FBXW7beta loss-of-function enhances FASN-mediated lipogenesis and promotes colorectal cancer growth. Signal Transduct Target Ther. 2023;8(1):187.
Google Scholar
Zhang H, Xia P, Yang Z, Liu J, Zhu Y, Huang Z, et al. Cullin-associated and neddylation-dissociated 1 regulate reprogramming of lipid metabolism through SKP1-Cullin-1-F-box(FBXO11) -mediated heterogeneous nuclear ribonucleoprotein A2/B1 ubiquitination and promote hepatocellular carcinoma. Clin Transl Med. 2023;13(10):e1443.
Google Scholar
Luo L, Wu X, Fan J, Dong L, Wang M, Zeng Y, et al. FBXO7 ubiquitinates PRMT1 to suppress Serine synthesis and tumor growth in hepatocellular carcinoma. Nat Commun. 2024;15(1):4790.
Google Scholar
Chavdoula E, Anastas V, La Ferlita A, Aldana J, Carota G, Spampinato M, et al. Transcriptional regulation of amino acid metabolism by KDM2B, in the context of ncPRC1.1 and in concert with MYC and ATF4. Metabolism. 2024;150:155719.
Google Scholar
Ferrer CM, Lynch TP, Sodi VL, Falcone JN, Schwab LP, Peacock DL, et al. O-GlcNAcylation regulates cancer metabolism and survival stress signaling via regulation of the HIF-1 pathway. Mol Cell. 2014;54(5):820–31.
Google Scholar
Zhang N, Meng Y, Mao S, Ni H, Huang C, Shen L, et al. FBXO31-mediated ubiquitination of OGT maintains O-GlcNAcylation homeostasis to restrain endometrial malignancy. Nat Commun. 2025;16(1):1274.
Google Scholar
Hayes JD, Dinkova-Kostova AT, Tew KD. Oxidative stress in cancer. Cancer Cell. 2020;38(2):167–97.
Google Scholar
Wang JY, Liu GZ, Wilmott JS, La T, Feng YC, Yari H, et al. Skp2-Mediated stabilization of MTH1 promotes survival of melanoma cells upon oxidative stress. Cancer Res. 2017;77(22):6226–39.
Google Scholar
Ji J, Jing A, Ding Y, Ma X, Qian Q, Geng T, et al. FBXO5-mediated RNF183 degradation prevents Endoplasmic reticulum stress-induced apoptosis and promotes colon cancer progression. Cell Death Dis. 2024;15(1):33.
Google Scholar
Li J, Zhao L, Wu Z, Huang S, Wang J, Chang Y, et al. SelK promotes glioblastoma cell proliferation by inhibiting beta-TrCP1 mediated ubiquitin-dependent degradation of CDK4. J Exp Clin Cancer Res. 2024;43(1):231.
Google Scholar
Mori A, Masuda K, Ohtsuka H, Shijo M, Ariake K, Fukase K, et al. FBXW7 modulates malignant potential and cisplatin-induced apoptosis in cholangiocarcinoma through NOTCH1 and MCL1. Cancer Sci. 2018;109(12):3883–95.
Google Scholar
Li N, Babaei-Jadidi R, Lorenzi F, Spencer-Dene B, Clarke P, Domingo E, et al. An FBXW7-ZEB2 axis links EMT and tumour microenvironment to promote colorectal cancer stem cells and chemoresistance. Oncogenesis. 2019;8(3):13.
Google Scholar
Liu J, Wei L, Miao Q, Zhan S, Chen P, Liu W, et al. MDM2 drives resistance to osimertinib by contextually disrupting FBW7-mediated destruction of MCL-1 protein in EGFR mutant NSCLC. J Exp Clin Cancer Res. 2024;43(1):302.
Google Scholar
Xiao Y, Yin C, Wang Y, Lv H, Wang W, Huang Y, et al. FBXW7 deletion contributes to lung tumor development and confers resistance to gefitinib therapy. Mol Oncol. 2018;12(6):883–95.
Google Scholar
Wu X, Iwatsuki M, Takaki M, Saito T, Hayashi T, Kondo M, et al. FBXW7 regulates the sensitivity of Imatinib in Gastrointestinal stromal tumors by targeting MCL1. Gastric Cancer. 2024;27(2):235–47.
Google Scholar
Richter KT, Kschonsak YT, Vodicska B, Hoffmann I. FBXO45-MYCBP2 regulates mitotic cell fate by targeting FBXW7 for degradation. Cell Death Differ. 2020;27(2):758–72.
Google Scholar
Salaroglio IC, Belisario DC, Bironzo P, Ananthanarayanan P, Ricci L, Digiovanni S, et al. SKP2 drives the sensitivity to neddylation inhibitors and cisplatin in malignant pleural mesothelioma. J Exp Clin Cancer Res. 2022;41(1):75.
Google Scholar
Lohmuller M, Roeck BF, Szabo TG, Schapfl MA, Pegka F, Herzog S, et al. The SKP2-p27 axis defines susceptibility to cell death upon CHK1 Inhibition. Mol Oncol. 2022;16(15):2771–87.
Google Scholar
Ren C, Han X, Lu C, Yang T, Qiao P, Sun Y, et al. Ubiquitination of NF-kappaB p65 by FBXW2 suppresses breast cancer stemness, tumorigenesis, and Paclitaxel resistance. Cell Death Differ. 2022;29(2):381–92.
Google Scholar
Zhu H, Wang X, Zhou X, Lu S, Gu G, Liu C. E3 ubiquitin ligase FBXW7 enhances radiosensitivity of non-small cell lung cancer cells by inhibiting SOX9 regulation of CDKN1A through ubiquitination. Lab Invest. 2022;102(11):1203–13.
Google Scholar
Cui D, Xiong X, Shu J, Dai X, Sun Y, Zhao Y. FBXW7 confers radiation survival by targeting p53 for degradation. Cell Rep. 2020;30(2):497–509. e4.
Google Scholar
Zhou Z, Zhang B, Deng Y, Deng S, Li J, Wei W, et al. FBW7/GSK3beta mediated degradation of IGF2BP2 inhibits IGF2BP2-SLC7A5 positive feedback loop and radioresistance in lung cancer. J Exp Clin Cancer Res. 2024;43(1):34.
Google Scholar
Chen Y, Zhou Y, Feng X, Wu Z, Yang Y, Rao X, et al. Targeting FBXO22 enhances radiosensitivity in non-small cell lung cancer by inhibiting the FOXM1/Rad51 axis. Cell Death Dis. 2024;15(1):104.
Google Scholar
Song Q, Wen J, Li W, Xue J, Zhang Y, Liu H, et al. HSP90 promotes radioresistance of cervical cancer cells via reducing FBXO6-mediated CD147 polyubiquitination. Cancer Sci. 2022;113(4):1463–74.
Google Scholar
Gstalder C, Liu D, Miao D, Lutterbach B, DeVine AL, Lin C, et al. Inactivation of Fbxw7 impairs DsRNA sensing and confers resistance to PD-1 Blockade. Cancer Discov. 2020;10(9):1296–311.
Google Scholar
Shen JZ, Qiu Z, Wu Q, Finlay D, Garcia G, Sun D, et al. FBXO44 promotes DNA replication-coupled repetitive element Silencing in cancer cells. Cell. 2021;184(2):352–69. e23.
Google Scholar
Liu W, Ren D, Xiong W, Jin X, Zhu L. A novel FBW7/NFAT1 axis regulates cancer immunity in sunitinib-resistant renal cancer by inducing PD-L1 expression. J Exp Clin Cancer Res. 2022;41(1):38.
Google Scholar
De S, Holvey-Bates EG, Mahen K, Willard B, Stark GR. The ubiquitin E3 ligase FBXO22 degrades PD-L1 and sensitizes cancer cells to DNA damage. Proc Natl Acad Sci U S A. 2021;118:47.
Zhang J, Lin XT, Yu HQ, Fang L, Wu D, Luo YD, et al. Elevated FBXL6 expression in hepatocytes activates VRK2-transketolase-ROS-mTOR-mediated immune evasion and liver cancer metastasis in mice. Exp Mol Med. 2023;55(10):2162–76.
Google Scholar
Lin XT, Zhang J, Liu ZY, Wu D, Fang L, Li CM, et al. Elevated FBXW10 drives hepatocellular carcinoma tumorigenesis via AR-VRK2 phosphorylation-dependent GAPDH ubiquitination in male Transgenic mice. Cell Rep. 2023;42(7):112812.
Google Scholar
Chan KL, Gomez J, Cardinez C, Kumari N, Sparbier CE, Lam EYN, et al. Inhibition of the CtBP complex and FBXO11 enhances MHC class II expression and anti-cancer immune responses. Cancer Cell. 2022;40(10):1190–206. e9.
Google Scholar
Tian T, Xie X, Yi W, Zhou Y, Xu Y, Wang Z, et al. FBXO38 mediates FGL1 ubiquitination and degradation to enhance cancer immunity and suppress inflammation. Cell Rep. 2023;42(11):113362.
Google Scholar
Meng X, Liu X, Guo X, Jiang S, Chen T, Hu Z, et al. FBXO38 mediates PD-1 ubiquitination and regulates anti-tumour immunity of T cells. Nature. 2018;564(7734):130–5.
Google Scholar
Shi Y, Zheng X, Peng H, Xu C, Sun R, Tian Z, et al. The E3 ubiquitin ligase FBXO38 maintains the antitumor function of natural killer cells by sustaining IL15R signaling. Cancer Immunol Res. 2024;12(10):1438–51.
Google Scholar
Harris R, Yang M, Schmidt C, Royet C, Singh S, Natarajan A et al. Fbxo7 promotes Cdk6 activity to inhibit PFKP and Glycolysis in T cells. J Cell Biol. 2022;221(7):e202203095.
Sun R, Lim SO. FBXL20-mediated ubiquitination triggers the proteasomal degradation of 4-1BB. FEBS J. 2022;289(15):4549–63.
Google Scholar
Li G, Wen Z, Xiong S. Microenvironmental beta-TrCP negates amino acid transport to trigger CD8(+) T cell exhaustion in human non-small cell lung cancer. Cell Rep. 2025;44(1):115128.
Google Scholar
Flugel D, Gorlach A, Kietzmann T. GSK-3beta regulates cell growth, migration, and angiogenesis via Fbw7 and USP28-dependent degradation of HIF-1alpha. Blood. 2012;119(5):1292–301.
Google Scholar
Kim YJ, Zhao Y, Myung JK, Yi JM, Kim MJ, Lee SJ. Suppression of breast cancer progression by FBXL16 via oxygen-independent regulation of HIF1alpha stability. Cell Rep. 2021;37(8):109996.
Google Scholar
Machado-Oliveira G, Guerreiro E, Matias AC, Facucho-Oliveira J, Pacheco-Leyva I, Braganca J. FBXL5 modulates HIF-1alpha transcriptional activity by degradation of CITED2. Arch Biochem Biophys. 2015;576:61–72.
Google Scholar
Lei Z, Luo Y, Lu J, Fu Q, Wang C, Chen Q, et al. FBXO22 promotes HCC angiogenesis and metastasis via RPS5/AKT/HIF-1alpha/VEGF-A signaling axis. Cancer Gene Ther. 2025;32(2):198–213.
Google Scholar
Zhang ZY, Sun JH, Liang MJ, Wang XP, Guan J, Zhou ZQ. The E3 ubiquitin ligase SCF (FBXW10)-mediated LATS2 degradation regulates angiogenesis and liver metastasis in colorectal cancer. Int J Biochem Cell Biol. 2023;158:106408.
Google Scholar
Shaik S, Nucera C, Inuzuka H, Gao D, Garnaas M, Frechette G, et al. SCF(beta-TRCP) suppresses angiogenesis and thyroid cancer cell migration by promoting ubiquitination and destruction of VEGF receptor 2. J Exp Med. 2012;209(7):1289–307.
Google Scholar
Dudley AC, Griffioen AW. Pathological angiogenesis: mechanisms and therapeutic strategies. Angiogenesis. 2023;26(3):313–47.
Google Scholar
Yin L, Zhang J, Zhu Z, Peng X, Lan H, Ayoub A, et al. The FBXW7-KMT2 axis in cancer-associated fibroblasts controls tumor growth via an epigenetic-paracrine mechanism. Proc Natl Acad Sci U S A. 2025;122(13):e2423130122.
Google Scholar
Chen JY, Li CF, Lai YS, Hung WC. Lysine demethylase 2A expression in cancer-associated fibroblasts promotes breast tumour growth. Br J Cancer. 2021;124(2):484–93.
Google Scholar
Hu JL, Wang W, Lan XL, Zeng ZC, Liang YS, Yan YR, et al. CAFs secreted exosomes promote metastasis and chemotherapy resistance by enhancing cell stemness and epithelial-mesenchymal transition in colorectal cancer. Mol Cancer. 2019;18(1):91.
Google Scholar
Yang N, Chen T, Wang L, Liu R, Niu Y, Sun L, et al. CXCR4 mediates matrix stiffness-induced downregulation of UBTD1 driving hepatocellular carcinoma progression via YAP signaling pathway. Theranostics. 2020;10(13):5790–801.
Google Scholar
Enriqué Steinberg JH, Rossi FA, Magliozzi R, Yuniati L, Santucci M, Rossi M, et al. SCF(βTrCP)-mediated degradation of SHARP1 in triple-negative breast cancer. Cell Death Dis. 2023;14(11):726.
Google Scholar
Li J, Zhao L, Wu Z, Huang S, Wang J, Chang Y, et al. SelK promotes glioblastoma cell proliferation by inhibiting β-TrCP1 mediated ubiquitin-dependent degradation of CDK4. J Experimental Clin Cancer Research: CR. 2024;43(1):231.
Google Scholar
Barik GK, Sahay O, Mukhopadhyay A, Manne RK, Islam S, Roy A, et al. FBXW2 suppresses breast tumorigenesis by targeting AKT-Moesin-SKP2 axis. Cell Death Dis. 2023;14(9):623.
Google Scholar
Yang F, Xu J, Li H, Tan M, Xiong X, Sun Y. FBXW2 suppresses migration and invasion of lung cancer cells via promoting β-catenin ubiquitylation and degradation. Nat Commun. 2019;10(1):1382.
Google Scholar
Zhou T, Chen T, Lai B, Zhang W, Luo X, Xia D, et al. FBXW2 inhibits prostate cancer proliferation and metastasis via promoting EGFR ubiquitylation and degradation. Cell Mol Life Sci. 2022;79(5):268.
Google Scholar
Xu F, Li J, Ni M, Cheng J, Zhao H, Wang S, et al. FBW7 suppresses ovarian cancer development by targeting the N(6)-methyladenosine binding protein YTHDF2. Mol Cancer. 2021;20(1):45.
Google Scholar
Pan Y, Liu J, Gao Y, Guo Y, Wang C, Liang Z, et al. FBXW7 loss of function promotes esophageal squamous cell carcinoma progression via elevating MAP4 and ERK phosphorylation. J Exp Clin Cancer Res. 2023;42(1):75.
Google Scholar
An HJ, Lee CJ, Lee GE, Choi Y, Jeung D, Chen W, et al. FBXW7-mediated ERK3 degradation regulates the proliferation of lung cancer cells. Exp Mol Med. 2022;54(1):35–46.
Google Scholar
Liu Y, Wang Q, Guo Q, Zhu Y, Lin L, Yang C, et al. FBXW7 directly ubiquitinates and degrades CTNNB1 mediating the suppression of ENKUR in endometrial cancer. Int J Biol Sci. 2025;21(4):1801–18.
Google Scholar
Lee CJ, An HJ, Kim SM, Yoo SM, Park J, Lee GE, et al. FBXW7-mediated stability regulation of signal transducer and activator of transcription 2 in melanoma formation. Proc Natl Acad Sci USA. 2020;117(1):584–94.
Google Scholar
Kourtis N, Moubarak RS, Aranda-Orgilles B, Lui K, Aydin IT, Trimarchi T, et al. FBXW7 modulates cellular stress response and metastatic potential through HSF1 post-translational modification. Nat Cell Biol. 2015;17(3):322–32.
Google Scholar
Huang LY, Zhao J, Chen H, Wan L, Inuzuka H, Guo J, et al. SCF(FBW7)-mediated degradation of Brg1 suppresses gastric cancer metastasis. Nat Commun. 2018;9(1):3569.
Google Scholar
Kuai X, Li L, Chen R, Wang K, Chen M, Cui B, et al. SCF(FBXW7)/GSK3β-Mediated GFI1 degradation suppresses proliferation of gastric cancer cells. Cancer Res. 2019;79(17):4387–98.
Google Scholar
Saffie R, Zhou N, Rolland D, Önder Ö, Basrur V, Campbell S, et al. FBXW7 triggers degradation of KMT2D to favor growth of diffuse large B-cell lymphoma cells. Cancer Res. 2020;80(12):2498–511.
Google Scholar
Zhang E, Chen S, Tang H, Fei C, Yuan Z, Mu X, et al. CDK1/FBXW7 facilitates degradation and ubiquitination of MLST8 to inhibit progression of renal cell carcinoma. Cancer Sci. 2022;113(1):91–108.
Google Scholar
Xiao G, Lu W, Yuan J, Liu Z, Wang P, Fan H. Fbxw7 suppresses carcinogenesis and stemness in triple-negative breast cancer through CHD4 degradation and Wnt/β-catenin pathway Inhibition. J Translational Med. 2024;22(1):99.
Google Scholar
Li C, Deng C, Pan G, Wang X, Zhang K, Dong Z, et al. Lycorine hydrochloride inhibits cell proliferation and induces apoptosis through promoting FBXW7-MCL1 axis in gastric cancer. J Experimental Clin Cancer Research: CR. 2020;39(1):230.
Google Scholar
Yao S, Xu F, Chen Y, Ge Y, Zhang F, Huang H, et al. Fbw7 regulates apoptosis in activated B-cell like diffuse large B-cell lymphoma by targeting Stat3 for ubiquitylation and degradation. J Experimental Clin Cancer Research: CR. 2017;36(1):10.
Google Scholar
Liu ZY, Lin XT, Zhang YJ, Gu YP, Yu HQ, Fang L, et al. FBXW10-S6K1 promotes ANXA2 polyubiquitination and KRAS activation to drive hepatocellular carcinoma development in males. Cancer Lett. 2023;566:216257.
Google Scholar
Yao J, Yang J, Yang Z, Wang XP, Yang T, Ji B, et al. FBXW11 contributes to stem-cell-like features and liver metastasis through regulating HIC1-mediated SIRT1 transcription in colorectal cancer. Cell Death Dis. 2021;12(10):930.
Google Scholar
Roberts JZ, Holohan C, Sessler T, Fox J, Crawford N, Riley JS, et al. The SCF(Skp2) ubiquitin ligase complex modulates TRAIL-R2-induced apoptosis by regulating FLIP(L). Cell Death Differ. 2020;27(9):2726–41.
Google Scholar
Wang J, Aldahamsheh O, Ferrena A, Borjihan H, Singla A, Yaguare S, et al. The interaction of SKP2 with p27 enhances the progression and stemness of osteosarcoma. Ann N Y Acad Sci. 2021;1490(1):90–104.
Google Scholar
Kuchay S, Giorgi C, Simoneschi D, Pagan J, Missiroli S, Saraf A, et al. PTEN counteracts FBXL2 to promote IP3R3- and Ca(2+)-mediated apoptosis limiting tumour growth. Nature. 2017;546(7659):554–8.
Google Scholar
Niu M, Xu J, Liu Y, Li Y, He T, Ding L, et al. FBXL2 counteracts Grp94 to destabilize EGFR and inhibit EGFR-driven NSCLC growth. Nat Commun. 2021;12(1):5919.
Google Scholar
Yao H, Su S, Xia D, Wang M, Li Z, Chen W et al. F-box and leucine-rich repeat protein 5 promotes colon cancer progression by modulating PTEN/PI3K/AKT signaling pathway. Biomedicine & pharmacotherapy = Biomedecine & pharmacotherapie. 2018;107:1712-9.
Li Y, Cui K, Zhang Q, Li X, Lin X, Tang Y, et al. FBXL6 degrades phosphorylated p53 to promote tumor growth. Cell Death Differ. 2021;28(7):2112–25.
Google Scholar
Shi W, Feng L, Dong S, Ning Z, Hua Y, Liu L, et al. FBXL6 governs c-MYC to promote hepatocellular carcinoma through ubiquitination and stabilization of HSP90AA1. Cell Commun Signal. 2020;18(1):100.
Google Scholar
Xiong HJ, Yu HQ, Zhang J, Fang L, Wu D, Lin XT, et al. Elevated FBXL6 activates both wild-type KRAS and mutant KRAS(G12D) and drives HCC tumorigenesis via the ERK/mTOR/PRELID2/ROS axis in mice. Military Med Res. 2023;10(1):68.
Google Scholar
Moro L, Simoneschi D, Kurz E, Arbini AA, Jang S, Guaragnella N, et al. Epigenetic Silencing of the ubiquitin ligase subunit FBXL7 impairs c-SRC degradation and promotes epithelial-to-mesenchymal transition and metastasis. Nat Cell Biol. 2020;22(9):1130–42.
Google Scholar
Yoshida A, Choi J, Jin HR, Li Y, Bajpai S, Qie S, et al. Fbxl8 suppresses lymphoma growth and hematopoietic transformation through degradation of Cyclin D3. Oncogene. 2021;40(2):292–306.
Google Scholar
Yang Y, Li S, Li B, Li Y, Xia K, Aman S, et al. FBXL10 promotes ERRα protein stability and proliferation of breast cancer cells by enhancing the mono-ubiquitylation of ERRα. Cancer Lett. 2021;502:108–19.
Google Scholar
Cui YH, Kim H, Lee M, Yi JM, Kim RK, Uddin N, et al. FBXL14 abolishes breast cancer progression by targeting CDCP1 for proteasomal degradation. Oncogene. 2018;37(43):5794–809.
Google Scholar
Fang X, Zhou W, Wu Q, Huang Z, Shi Y, Yang K, et al. Deubiquitinase USP13 maintains glioblastoma stem cells by antagonizing FBXL14-mediated Myc ubiquitination. J Exp Med. 2017;214(1):245–67.
Google Scholar
Morel M, Long W. FBXL16 promotes cell growth and drug resistance in lung adenocarcinomas with KRAS mutation by stabilizing IRS1 and upregulating IRS1/AKT signaling. Mol Oncol. 2024;18(3):762–77.
Google Scholar
Zhang J, Yang Z, Ou J, Xia X, Zhi F, Cui J. The F-box protein FBXL18 promotes glioma progression by promoting K63-linked ubiquitination of Akt. FEBS Lett. 2017;591(1):145–54.
Google Scholar
Yu HQ, Li F, Xiong H, Fang L, Zhang J, Bie P et al. Elevated FBXL18 promotes RPS15A ubiquitination and SMAD3 activation to drive HCC. Hepatology communications. 2023;7(7):e00198.
Manne RK, Agrawal Y, Malonia SK, Banday S, Edachery S, Patel A, et al. FBXL20 promotes breast cancer malignancy by inhibiting apoptosis through degradation of PUMA and BAX. J Biol Chem. 2021;297(4):101253.
Google Scholar
Lee GE, Jeung D, Chen W, Byun J, Lee JY, Kang HC, et al. MEKs/ERKs-mediated FBXO1/E2Fs interaction interference modulates G(1)/S cell cycle transition and cancer cell proliferation. Arch Pharm Res. 2023;46(1):44–58.
Google Scholar
Ji J, Shen J, Xu Y, Xie M, Qian Q, Qiu T, et al. FBXO2 targets glycosylated SUN2 for ubiquitination and degradation to promote ovarian cancer development. Cell Death Dis. 2022;13(5):442.
Google Scholar
Che X, Jian F, Wang Y, Zhang J, Shen J, Cheng Q, et al. FBXO2 promotes proliferation of endometrial cancer by Ubiquitin-Mediated degradation of FBN1 in the regulation of the cell cycle and the autophagy pathway. Front Cell Dev Biology. 2020;8:843.
Qie S, Majumder M, Mackiewicz K, Howley BV, Peterson YK, Howe PH, et al. Fbxo4-mediated degradation of Fxr1 suppresses tumorigenesis in head and neck squamous cell carcinoma. Nat Commun. 2017;8(1):1534.
Google Scholar
Feng C, Yang F, Wang J. FBXO4 inhibits lung cancer cell survival by targeting Mcl-1 for degradation. Cancer Gene Ther. 2017;24(8):342–7.
Google Scholar
Ji M, Zhao Z, Li Y, Xu P, Shi J, Li Z, et al. FBXO6-mediated RNASET2 ubiquitination and degradation governs the development of ovarian cancer. Cell Death Dis. 2021;12(4):317.
Google Scholar
Zhang H, Zhao Y, Wang J, Li J, Xia J, Lin Y, et al. FBXO7, a tumor suppressor in endometrial carcinoma, suppresses INF2-associated mitochondrial division. Cell Death Dis. 2023;14(6):368.
Google Scholar
FeiFei W, HongHai X, YongRong Y, PingXiang W, JianHua W, XiaoHui Z, et al. FBX8 degrades GSTP1 through ubiquitination to suppress colorectal cancer progression. Cell Death Dis. 2019;10(5):351.
Google Scholar
Wang FF, Zhang XJ, Yan YR, Zhu XH, Yu J, Ding Y, et al. FBX8 is a metastasis suppressor downstream of miR-223 and targeting mTOR for degradation in colorectal carcinoma. Cancer Lett. 2017;388:85–95.
Google Scholar
Xuan Z, Chen C, Sun H, Yang K, Li J, Fu M, et al. NDR1/FBXO11 promotes phosphorylation-mediated ubiquitination of β-catenin to suppress metastasis in prostate cancer. Int J Biol Sci. 2024;20(12):4957–77.
Google Scholar
Pighi C, Cheong TC, Compagno M, Patrucco E, Arigoni M, Olivero M, et al. Frequent mutations of FBXO11 highlight BCL6 as a therapeutic target in Burkitt lymphoma. Blood Adv. 2021;5(23):5239–57.
Google Scholar
Paul D, Islam S, Manne RK, Dinesh US, Malonia SK, Maity B, et al. F-box protein FBXO16 functions as a tumor suppressor by attenuating nuclear β-catenin function. J Pathol. 2019;248(3):266–79.
Google Scholar
Ji M, Zhao Z, Li Y, Xu P, Shi J, Li Z, et al. FBXO16-mediated HnRNPL ubiquitination and degradation plays a tumor suppressor role in ovarian cancer. Cell Death Dis. 2021;12(8):758.
Google Scholar
Dobish KK, Wittorf KJ, Swenson SA, Bean DC, Gavile CM, Woods NT, et al. FBXO21 mediated degradation of p85α regulates proliferation and survival of acute myeloid leukemia. Leukemia. 2023;37(11):2197–208.
Google Scholar
Ge MK, Zhang N, Xia L, Zhang C, Dong SS, Li ZM, et al. FBXO22 degrades nuclear PTEN to promote tumorigenesis. Nat Commun. 2020;11(1):1720.
Google Scholar
Zhu XN, He P, Zhang L, Yang S, Zhang HL, Zhu D, et al. FBXO22 mediates polyubiquitination and inactivation of LKB1 to promote lung cancer cell growth. Cell Death Dis. 2019;10(7):486.
Google Scholar
Lignitto L, LeBoeuf SE, Homer H, Jiang S, Askenazi M, Karakousi TR, et al. Nrf2 activation promotes lung cancer metastasis by inhibiting the degradation of Bach1. Cell. 2019;178(2):316-329.e18.
Google Scholar
Zhang H, Bai Y, Li J, Chen T, Shang G. FBXO22 promotes osteosarcoma progression via regulation of FOXO1 for ubiquitination and degradation. J Cell Mol Med. 2024;28(16):e70021.
Google Scholar
Bai J, Wu K, Cao MH, Yang Y, Pan Y, Liu H, et al. SCF(FBXO22) targets HDM2 for degradation and modulates breast cancer cell invasion and metastasis. Proc Natl Acad Sci USA. 2019;116(24):11754–63.
Google Scholar
Liu P, Cong X, Liao S, Jia X, Wang X, Dai W, et al. Global identification of phospho-dependent SCF substrates reveals a FBXO22 phosphodegron and an ERK-FBXO22-BAG3 axis in tumorigenesis. Cell Death Differ. 2022;29(1):1–13.
Google Scholar
Zhu XN, Wei YS, Yang Q, Liu HR, Zhi Z, Zhu D, et al. FBXO22 promotes leukemogenesis by targeting BACH1 in MLL-rearranged acute myeloid leukemia. J Hematol Oncol. 2023;16(1):9.
Google Scholar
Nie DY, Tabor JR, Li J, Kutera M, St-Germain J, Hanley RP, et al. Recruitment of FBXO22 for targeted degradation of NSD2. Nat Chem Biol. 2024;20(12):1597–607.
Google Scholar
Lin M, Zhang J, Bouamar H, Wang Z, Sun LZ, Zhu X. Fbxo22 promotes cervical cancer progression via targeting p57(Kip2) for ubiquitination and degradation. Cell Death Dis. 2022;13(9):805.
Google Scholar
Zhang L, Chen J, Ning D, Liu Q, Wang C, Zhang Z, et al. FBXO22 promotes the development of hepatocellular carcinoma by regulating the ubiquitination and degradation of p21. J Experimental Clin Cancer Research: CR. 2019;38(1):101.
Google Scholar
Chen K, Wang Y, Dai X, Luo J, Hu S, Zhou Z, et al. FBXO31 is upregulated by METTL3 to promote pancreatic cancer progression via regulating SIRT2 ubiquitination and degradation. Cell Death Dis. 2024;15(1):37.
Google Scholar
Duan S, Moro L, Qu R, Simoneschi D, Cho H, Jiang S, et al. Loss of FBXO31-mediated degradation of DUSP6 dysregulates ERK and PI3K-AKT signaling and promotes prostate tumorigenesis. Cell Rep. 2021;37(3):109870.
Google Scholar
Su D, Wang R, Chen G, Ding C, Liu Y, Tao J, et al. FBXO32 stimulates protein synthesis to drive pancreatic cancer progression and metastasis. Cancer Res. 2024;84(16):2607–25.
Google Scholar
Wu J, Wen T, Marzio A, Song D, Chen S, Yang C, et al. FBXO32-mediated degradation of PTEN promotes lung adenocarcinoma progression. Cell Death Dis. 2024;15(4):282.
Google Scholar
Zhang N, Liao Y, Lv W, Zhu S, Qiu Y, Chen N, et al. FBXO32 targets PHPT1 for ubiquitination to regulate the growth of EGFR mutant lung cancer. Cell Oncol (Dordrecht Netherlands). 2022;45(2):293–307.
Google Scholar
Zhou H, Liu Y, Zhu R, Ding F, Wan Y, Li Y, et al. FBXO32 suppresses breast cancer tumorigenesis through targeting KLF4 to proteasomal degradation. Oncogene. 2017;36(23):3312–21.
Google Scholar
Wu Z, Peng Y, Chen W, Xia F, Song T, Ke Q. Lactylation-driven transcriptional activation of FBXO33 promotes gallbladder cancer metastasis by regulating p53 polyubiquitination. Cell Death Dis. 2025;16(1):144.
Google Scholar
Zheng L, Shen J, Chen Y, Lin J, Li P, Zhao X, et al. FBXO43 promotes cell cycle progression in cancer cells through stabilizing SKP2. Cancer Lett. 2024;591:216848.
Google Scholar
Lin XT, Yu HQ, Fang L, Tan Y, Liu ZY, Wu D et al. Elevated FBXO45 promotes liver tumorigenesis through enhancing IGF2BP1 ubiquitination and subsequent PLK1 upregulation. eLife. 2021;10:e70715.
Wang Q, Xu C, Cai R, An W, Yuan H, Xu M. Fbxo45-mediated NP-STEP(46) degradation via K6-linked ubiquitination sustains ERK activity in lung cancer. Mol Oncol. 2022;16(16):3017–33.
Google Scholar
Zheng M, Wu L, Xiao R, Cai J, Chen W, Shen S. Fbxo45 facilitates the malignant progression of breast cancer by targeting Bim for ubiquitination and degradation. BMC Cancer. 2024;24(1):619.
Google Scholar
Wu L, Yu K, Chen K, Zhu X, Yang Z, Wang Q, et al. Fbxo45 facilitates pancreatic carcinoma progression by targeting USP49 for ubiquitination and degradation. Cell Death Dis. 2022;13(3):231.
Google Scholar
Xie W, Jiang Q, Wu X, Wang L, Gao B, Sun Z, et al. IKBKE phosphorylates and stabilizes snail to promote breast cancer invasion and metastasis. Cell Death Differ. 2022;29(8):1528–40.
Google Scholar
Li F, Niu M, Qin K, Guo R, Yi Y, Xu J, et al. FBXL2 promotes E47 protein instability to inhibit breast cancer stemness and Paclitaxel resistance. Oncogene. 2023;42(5):339–50.
Google Scholar
Yeh CH, Bellon M, Wang F, Zhang H, Fu L, Nicot C. Loss of FBXW7-mediated degradation of BRAF elicits resistance to BET inhibitors in adult T cell leukemia cells. Mol Cancer. 2020;19(1):139.
Google Scholar
Patel A, Garcia LF, Mannella V, Gammon L, Borg TM, Maffucci T, et al. Targeting p63 upregulation abrogates resistance to MAPK inhibitors in melanoma. Cancer Res. 2020;80(12):2676–88.
Google Scholar
Park S, Ryu WJ, Kim TY, Hwang Y, Han HJ, Lee JD, et al. Overcoming BRAF and CDK4/6 inhibitor resistance by inhibiting MAP3K3-dependent protection against YAP lysosomal degradation. Exp Mol Med. 2024;56(4):987–1000.
Google Scholar
Zhou Z, Zhang B, Deng Y, Deng S, Li J, Wei W, et al. FBW7/GSK3β mediated degradation of IGF2BP2 inhibits IGF2BP2-SLC7A5 positive feedback loop and radioresistance in lung cancer. J Experimental Clin Cancer Research: CR. 2024;43(1):34.
Google Scholar
Continue Reading
-

Starting 5, October 13: Top Picks Take Center Stage
Tonight, the NBA world is set for a look at the top 3 picks from this year’s Draft.
Last night? It belonged to Olivier Sarr — older brother of 2024 No. 2 pick Alex — who got the best of their family duel on Sunday with this…
Continue Reading
-

1 Day Maximising Value and Successfully Protecting Your
Dublin, Oct. 13, 2025 (GLOBE NEWSWIRE) — The “Maximising Value and Successfully Protecting Your Trade Mark Training Course (Nov 3, 2025)” training has been added to ResearchAndMarkets.com’s offering.
In today’s brand-driven world, trade…
Continue Reading
-
Just a moment…
Just a moment… This request seems a bit unusual, so we need to confirm that you’re human. Please press and hold the button until it turns completely green. Thank you for your cooperation!
Continue Reading
-

Quote & Analysis: Magic’s Unselfishness, Franz’s Free Throw Rate, Jase’s 3-Point Shooting & More – NBA
- Quote & Analysis: Magic’s Unselfishness, Franz’s Free Throw Rate, Jase’s 3-Point Shooting & More NBA
- Orlando Magic continue putting biggest weakness to rest Orlando Magic Daily
- 2025-26 NBA Guide- Orlando Magic The Playoffs
- Orlando Magic…
Continue Reading
-
Live updates: Trump declares ‘dawn of a new Middle East,’ celebrating release of all living hostages – The Washington Post
- Live updates: Trump declares ‘dawn of a new Middle East,’ celebrating release of all living hostages The Washington Post
- LIVE: After Israel, Trump arrives in Egypt for Gaza ceasefire summit Al Jazeera
- Next 24 hours: Three things to watch on…
Continue Reading
-
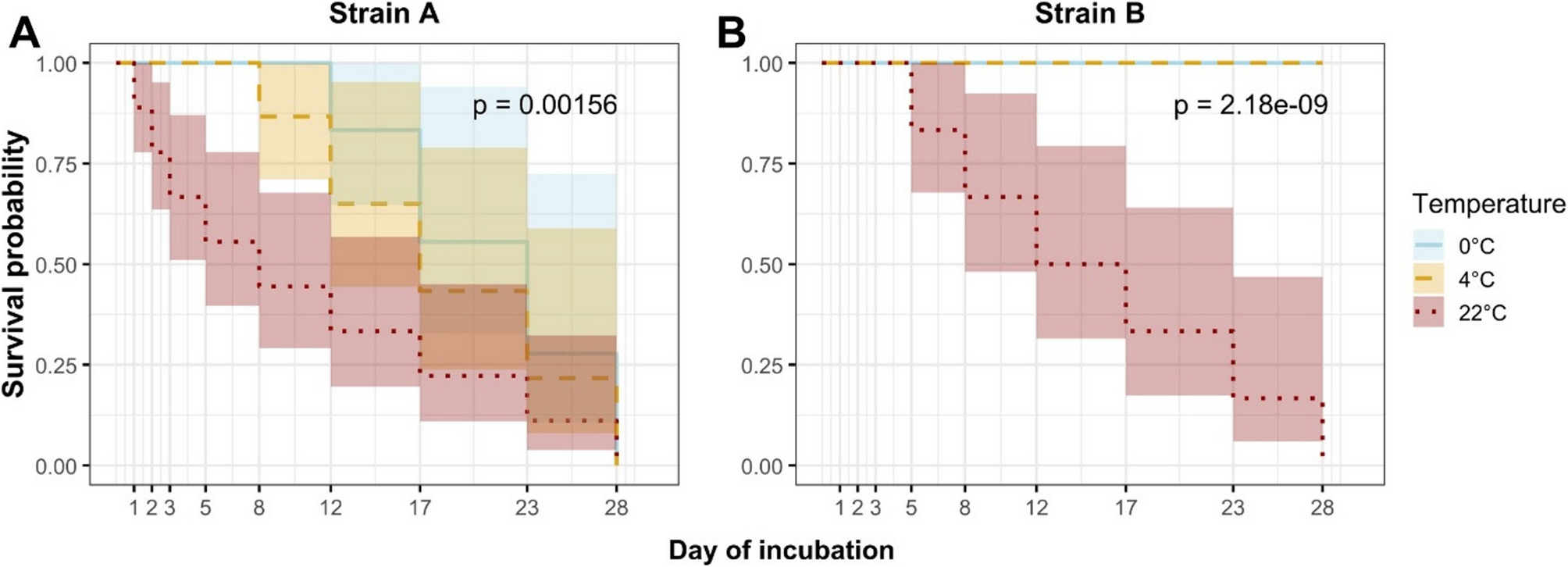
Temperature-dependent survival of Mycoplasma anserisalpingitidis in water: implications for biosecurity and transmission in waterfowl farming | BMC Veterinary Research
Ferguson-Noel N, Armour NK, Noormohammadi AH, El-Gazzar M, Bradbury JM. Mycoplasmosis. In: Swayne DE, editor. Diseases of Poultry. 14th ed. Hoboken, NJ, USA: John Wiley & Sons, Inc.; 2020. p. 907–65.
Volokhov DV, Grózner D, Gyuranecz M, Ferguson-Noel N, Gao Y, Bradbury JM, et al. Mycoplasma anserisalpingitidis sp. nov., isolated from European domestic geese (Anser anser domesticus) with reproductive pathology. Int J Syst Evol Microbiol. 2020;70:2369–81. https://doi.org/10.1099/ijsem.0.004052.
Google Scholar
Sawicka-Durkalec A, Tomczyk G, Kursa O, Stenzel T, Gyuranecz M. Evidence of Mycoplasma spp. Transmission by migratory wild geese. Poult Sci. 2022;101:101526. https://doi.org/10.1016/j.psj.2021.101526.
Google Scholar
Polak-Vogelzang AA. Survival of Mycoplasma gallisepticum in mains water. Avian Pathol. 1977;6:93–5. https://doi.org/10.1080/03079457708418215.
Google Scholar
Marois C, Savoye C, Kobisch M, Kempf I. A reverse transcription-PCR assay to detect viable Mycoplasma synoviae in poultry environmental samples. Vet Microbiol. 2002;89:17–28. https://doi.org/10.1016/S0378-1135(02)00159-1.
Google Scholar
Marois C, Dufour-Gesbert F, Kempf I. Polymerase chain reaction for detection of Mycoplasma gallisepticum in environmental samples. Avian Pathol. 2002;31:163–8. https://doi.org/10.1080/03079450120118658.
Google Scholar
Marois C, Picault J-P, Kobisch M, Kempf I. Experimental evidence of indirect transmission of Mycoplasma synoviae. Vet Res. 2005;36:759–69. https://doi.org/10.1051/vetres:2005031.
Google Scholar
Münster P, Kemper N. Long-term analysis of drinking water quality in poultry and pig farms in Northwest Germany. Front Anim Sci. 2024;5:1467287. https://doi.org/10.3389/fanim.2024.1467287.
Google Scholar
Elmberg J, Berg C, Lerner H, Waldenström J, Hessel R. Potential disease transmission from wild geese and swans to livestock, poultry and humans : a review of the scientific literature from a one health perspective. Infect Ecol Epidemiol. 2017;7. https://doi.org/10.1080/20008686.2017.1300450.
Abulreesh HH, Paget TA, Goulder R. Waterfowl and the bacteriological quality of amenity ponds. J Water Health. 2004;2:183–9. https://doi.org/10.2166/wh.2004.0016.
Google Scholar
Gonzalez JM, Aranda B. Microbial growth under limiting Conditions-Future perspectives. Microorganisms. 2023;11:1641. https://doi.org/10.3390/microorganisms11071641.
Google Scholar
Arana I, Muela A, Orruño M, Seco C, Garaizabal I, Barcina I. Effect of temperature and starvation upon survival strategies of Pseudomonas fluorescens CHA0: comparison with Escherichia coli. FEMS Microbiol Ecol. 2010;74:500–9. https://doi.org/10.1111/j.1574-6941.2010.00979.x.
Google Scholar
Nedwell DB. Effect of low temperature on microbial growth: Lowered affinity for substrates limits growth at low temperature. FEMS Microbiol Ecol. 2006;30:101–11. https://doi.org/10.1111/j.1574-6941.1999.tb00639.x.
Google Scholar
Marmion M, Macori G, Ferone M, Whyte P, Scannell AGM. Survive and thrive: control mechanisms that facilitate bacterial adaptation to survive manufacturing-related stress. Int J Food Microbiol. 2022;368:109612. https://doi.org/10.1016/j.ijfoodmicro.2022.109612.
Google Scholar
Moon S, Ham S, Jeong J, Ku H, Kim H, Lee C. Temperature matters: bacterial response to temperature change. J Microbiol. 2023;61:343–57. https://doi.org/10.1007/s12275-023-00031-x.
Google Scholar
Citti C, Blanchard A. Mycoplasmas and their host: emerging and re-emerging minimal pathogens. Trends Microbiol. 2013;21:196–203. https://doi.org/10.1016/j.tim.2013.01.003.
Google Scholar
Rocha EPC, Blanchard A. Genomic repeats, genome plasticity and the dynamics of Mycoplasma evolution. Nucleic Acids Res. 2002;30:2031–42. https://doi.org/10.1093/nar/30.9.2031.
Google Scholar
Katz SD. The Streak Plate Protocol. Am Soc Microbiol. 2008;1–10. https://asm.org/asm/media/protocol-images/the-streak-plate-protocol.pdf.
Grózner D, Sulyok KM, Kreizinger Z, Rónai Z, Jánosi S, Turcsányi I, et al. Detection of Mycoplasma anatis, M. anseris, M. cloacale and Mycoplasma sp. 1220 in waterfowl using species-specific PCR assays. PLoS ONE. 2019;14:e0219071. https://doi.org/10.1371/journal.pone.0219071.
Google Scholar
Gioia G, Werner B, Nydam DV, Moroni P. Validation of a Mycoplasma molecular diagnostic test and distribution of Mycoplasma species in bovine milk among new York state dairy farms. J Dairy Sci. 2016;99:4668–77. https://doi.org/10.3168/jds.2015-10724.
Google Scholar
Hannan PCT. Guidelines and recommendations for antimicrobial minimum inhibitory concentration (MIC) testing against veterinary Mycoplasma species. Vet Res. 2000;31:373–95. https://doi.org/10.1051/vetres:2000100.
Google Scholar
Bekő K, Grózner D, Mitter A, Udvari L, Földi D, Wehmann E, et al. Development and evaluation of temperature-sensitive Mycoplasma anserisalpingitidis clones as vaccine candidates. Avian Pathol. 2022;51:535–49. https://doi.org/10.1080/03079457.2022.2102967.
Google Scholar
Terry M. Therneau, Patricia M. Grambsch. Modeling Survival Data: Extending the Cox Model. New York: Springer; 2000.
Tang Y, Horikoshi M, Li W. Ggfortify: unified interface to visualize statistical results of popular R packages. R J. 2016;8:474. https://doi.org/10.32614/RJ-2016-060.
Google Scholar
Wickham H. ggplot2: Elegant Graphics for Data Analysis. New York: Springer-Verlag; 2016. https://doi.org/10.1007/978-3-319-24277-4.
R Core Team. R: A Language and environment for statistical computing. Vienna, Austria: Foundation for Statistical Computing; 2025.
Posit team. RStudio: Integrated Development Environment for R. Posit Software, PBC, Boston: MA; 2025. http://www.posit.co/.
Merchant SS, Helmann JD. Elemental economy: microbial strategies for optimizing growth in the face of nutrient limitation. Adv Microb Physiol. 2012;91–210. https://doi.org/10.1016/B978-0-12-398264-3.00002-4.
Nagatomo H. Comparative studies of the persistence of animal Mycoplasmas under different environmental conditions. Vet Microbiol. 2001;82:223–32. https://doi.org/10.1016/S0378-1135(01)00385-6.
Google Scholar
Justice-Allen A, Trujillo J, Corbett R, Harding R, Goodell G, Wilson D. Survival and replication of Mycoplasma species in recycled bedding sand and association with mastitis on dairy farms in Utah. J Dairy Sci. 2010;93:192–202. https://doi.org/10.3168/jds.2009-2474.
Google Scholar
Nouvel LX, Sirand-Pugnet P, Marenda MS, Sagné E, Barbe V, Mangenot S, et al. Comparative genomic and proteomic analyses of two Mycoplasma agalactiae strains: clues to the macro- and micro-events that are shaping Mycoplasma diversity. BMC Genomics. 2010;11:86. https://doi.org/10.1186/1471-2164-11-86.
Google Scholar
Delaney NF, Balenger S, Bonneaud C, Marx CJ, Hill GE, Ferguson-Noel N, et al. Ultrafast evolution and loss of crisprs following a host shift in a novel wildlife pathogen, Mycoplasma gallisepticum. PLoS Genet. 2012;8:e1002511. https://doi.org/10.1371/journal.pgen.1002511.
Google Scholar
Bekő K, Nagy EZ, Grózner D, Kreizinger Z, Gyuranecz M. Biofilm formation and its impact on environmental survival and antibiotic resistance of Mycoplasma anserisalpingitidis strains. Acta Vet Hung. 2022;70:184–91. https://doi.org/10.1556/004.2022.00029.
Google Scholar
Rossi C, Chaves-López C, Serio A, Goffredo E, Cenci Goga BT, Paparella A. Influence of incubation conditions on biofilm formation by Pseudomonas fluorescens isolated from dairy products and dairy manufacturing plants. Ital J Food Saf. 2016;5. https://doi.org/10.4081/ijfs.2016.5793.
De Plano LM, Caratozzolo M, Conoci S, Guglielmino SPP, Franco D. Impact of nutrient starvation on biofilm formation in Pseudomonas aeruginosa: an analysis of growth, adhesion, and Spatial distribution. Antibiotics. 2024;13:987. https://doi.org/10.3390/antibiotics13100987.
Google Scholar
Catania S, Bottinelli M, Fincato A, Tondo A, Matucci A, Nai G, et al. Pathogenic avian Mycoplasmas show phenotypic differences in their biofilm forming ability compared to non-pathogenic species in vitro. Biofilm. 2024;7:100190. https://doi.org/10.1016/j.bioflm.2024.100190.
Google Scholar
Pletnev P, Osterman I, Sergiev P, Bogdanov A, Dontsova O. Survival guide: Escherichia coli in the stationary phase. Acta Naturae. 2015;7:22–33.
Google Scholar
Navarro Llorens JM, Tormo A, Martínez-García E. Stationary phase in gram-negative bacteria. FEMS Microbiol Rev. 2010;34:476–95. https://doi.org/10.1111/j.1574-6976.2010.00213.x.
Google Scholar
Hazan R, Schoemann M, Klutstein M. Endurance of extremely prolonged nutrient prevention across kingdoms of life. iScience. 2021;24:102745. https://doi.org/10.1016/j.isci.
Google Scholar
Chernov VM, Gogolev YV, Mukhametshina NE, Abdrakhimov FA, Chernova OA. Mycoplasma adaptation to biogenic and abiogenic stessful factors; Acholeplasma laidlawii nannotransformation and minibodies. Prog Nucl Energy 6 Biol Sci. 2003;396:417–20. https://doi.org/10.0012/4966/04/0506-0251.
Demina IA, Serebryakova MV, Ladygina VG, Rogova MA, Kondratov IG, Renteeva AN, et al. Proteomic characterization of Mycoplasma gallisepticum nanoforming. Biochem (Moscow). 2010;75:1252–7. https://doi.org/10.1134/S0006297910100068.
Google Scholar
Chernov VM, Chernova OA, Gorshkov OV, Muzykantov AA, Shaimardanova GF, Pel’nikevich AD, et al. Adaptation of Mycoplasma gallisepticum to unfavorable growth conditions: changes in morphological and physiological characteristics. Microbiol (N Y). 2008;77:691–4. https://doi.org/10.1134/S0026261708060064.
Google Scholar
Chernov VM, Chernova OA, Medvedeva ES, Sorvina AI, Davydova MN, Rogova MA, et al. Responses of Acholeplasma Laidlawii PG8 cells to cold shock and oxidative stress: proteomic analysis and stress-reactive Mycoplasma proteins. Dokl Biochem Biophys. 2010;432:126–30. https://doi.org/10.1134/S1607672910030099.
Google Scholar
Piccirillo A, Tolosi R, Mughini-Gras L, Kers JG, Laconi A. Drinking water and biofilm as sources of antimicrobial resistance in Free-Range organic broiler farms. Antibiotics. 2024;13:808. https://doi.org/10.3390/antibiotics13090808.
Google Scholar
Mustedanagic A, Matt M, Weyermair K, Schrattenecker A, Kubitza I, Firth CL, et al. Assessment of microbial quality in poultry drinking water on farms in Austria. Front Vet Sci. 2023;10:1254442. https://doi.org/10.3389/fvets.2023.1254442.
Google Scholar
Kapperud G, Skjerve E, Vik L, Hauge K, Lysaker A, Aalmen I, et al. Epidemiological investigation of risk factors for Campylobacter colonization in Norwegian broiler flocks. Epidemiol Infect. 1993;111:245–55. https://doi.org/10.1017/s0950268800056958.
Google Scholar
Sparks NHC. The role of the water supply system in the infection and control of Campylobacter in chicken. Worlds Poult Sci J. 2009;65:459–74. https://doi.org/10.1017/S0043933909000324.
Google Scholar
Gbylik-Sikorska M, Posyniak A, Sniegocki T, Sell B, Gajda A, Sawicka A, et al. Influence of enrofloxacin traces in drinking water to doxycycline tissue pharmacokinetics in healthy and infected by Mycoplasma gallisepticum broiler chickens. Food Chem Toxicol. 2016;90:123–9. https://doi.org/10.1016/j.fct.2016.02.006.
Google Scholar
Continue Reading
-

Sugary and sweetened drinks increase liver disease risk
Are sweetened drinks worse than sugary ones? Key Liver Risk Summary
- Sweetened drinks raise liver disease risk by 60 percent daily intake
- Sugary drinks increase liver disease risk by 50 percent per day
- Sweetened drinks linked to liver-related deaths,…
Continue Reading
