- Human Embryo Model Produces Blood Stem Cells Inside Precision Medicine
- Cambridge lab-grown human embryo model produces blood cells BBC
- Breakthrough in lab-grown blood cells could pave way for regenerative therapies PA Media
- Lab grown embryo…
Blog
-
Human Embryo Model Produces Blood Stem Cells – Inside Precision Medicine
-
Just a moment…
Just a moment… This request seems a bit unusual, so we need to confirm that you’re human. Please press and hold the button until it turns completely green. Thank you for your cooperation!
Continue Reading
-
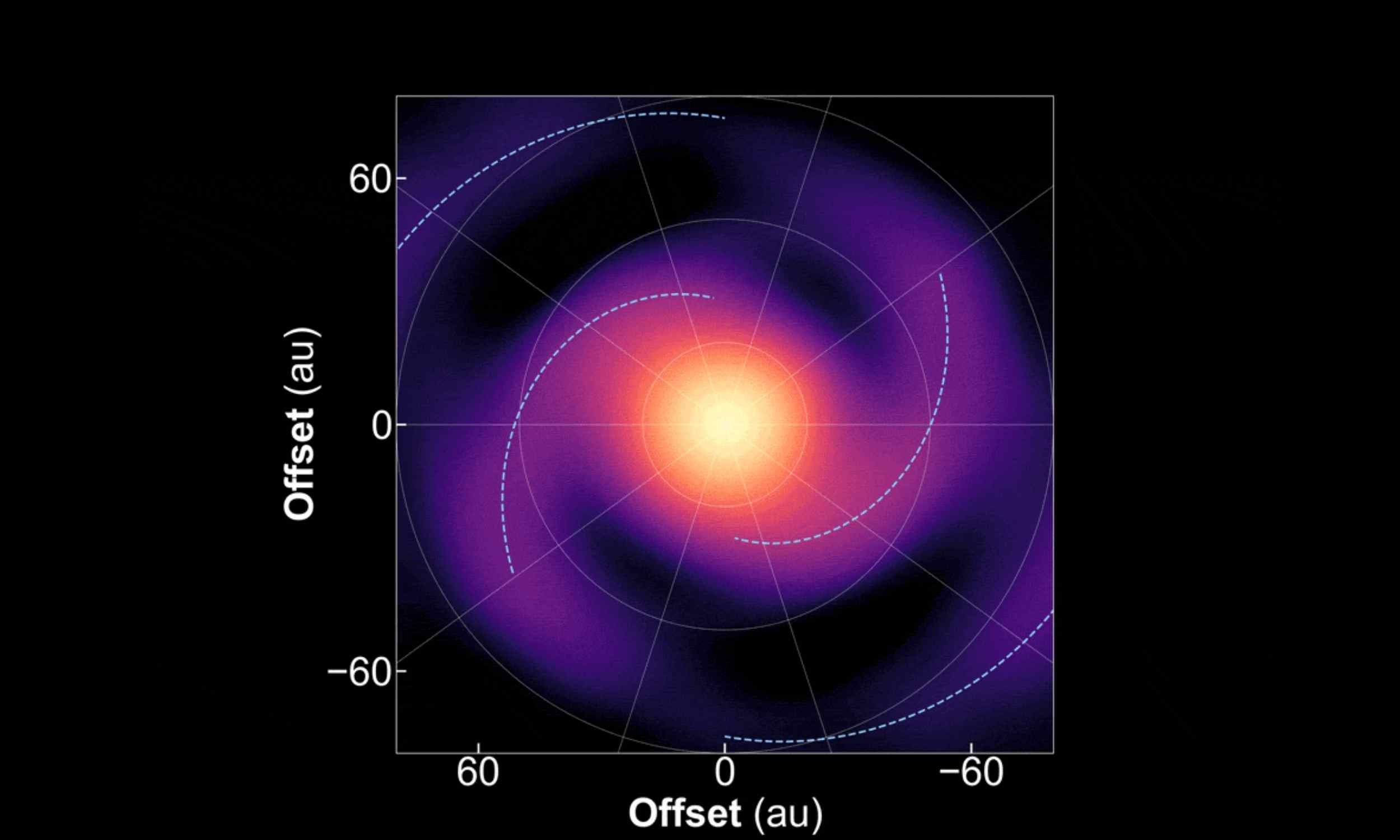
ALMA captures spiral motion that gives rise to new planets
A peer-reviewed study shows spiral patterns in a young star’s disk actually moving over time. The array turned snapshots into a short view that reveals their winding motion.
The star, called IM Lup, lies in the constellation Lupus. Using data…
Continue Reading
-

How to watch and follow Atletico Madrid v Man Utd Women in UEFA Champions League | 16 October 2025
HOW THE COMPETITION WORKS
The Women’s Champions League format for this season is slightly different to 2024/25, following the same structure adopted in the men’s game last term.
Instead of a 16-team group stage split into four pools, this season…
Continue Reading
-

Honor is making a smartphone with a fold-out camera
Device maker Honor has revealed that one of its upcoming smartphones will offer an unusual design choice. While many manufacturers push to have more numerous and powerful cameras in their products, the Chinese company will put the camera for its…
Continue Reading
-

Gwyneth Paltrow Says Biography Is ‘Rubbish,’ ‘Sexist’
Gwyneth Paltrow is addressing Gwyneth: The Biography, Amy Odell’s July book about her life, which she had no involvement in.
In a Wednesday profile with British Vogue, the Oscar winner was asked about the biography, which features over…
Continue Reading
-

Daniels’ Event Film Back on Universal Calendar for 2027
The anticipated Everything Everywhere All at Once followup from Oscar winning duo Daniels is back on the Universal calendar.
The untitled feature will hit theaters on Nov. 19, 2027. The feature previously was set for…
Continue Reading
-
Just a moment…
Just a moment… This request seems a bit unusual, so we need to confirm that you’re human. Please press and hold the button until it turns completely green. Thank you for your cooperation!
Continue Reading
-
Just a moment…
Just a moment… This request seems a bit unusual, so we need to confirm that you’re human. Please press and hold the button until it turns completely green. Thank you for your cooperation!
Continue Reading
-

Sebela Women’s Health’s MIUDELLA® Hormone-Free Copper Intrauterine System (IUS) Named to TIME’s List of the Best Inventions of 2025
ROSWELL, Ga., Oct. 15, 2025 /PRNewswire/ — Sebela Women’s Health Inc., a part of Sebela Pharmaceuticals, today announced that MIUDELLA® Hormone-Free Copper Intrauterine System (IUS) has been named to TIME’s list of the best inventions of 2025.
To compile this year’s list, TIME solicited nominations from TIME editors and correspondents around the world through an online application process, paying special attention to growing fields—such as health care and AI. TIME then evaluated each contender on a number of key factors, including originality, efficacy, ambition, and impact. See the full list here: time.com/collections/best-inventions-2025/ and MIUDELLA® brief here: https://time.com/collections/best-inventions-2025/7318454/sebela-miudella/.
MIUDELLA® is the first hormone-free copper IUD in the U.S. in over 40 years. It was approved on February 24, 2025, by the U.S. Food and Drug Administration for the prevention of pregnancy in females of reproductive potential for up to three years, and it is expected to be available to patients through trained healthcare providers in the U.S. in the first half of 2026.
“Sebela Women’s Health is delighted that MIUDELLA was named to TIME’s Best Inventions of 2025 list,” said Kelly Culwell, MD, Head of Research and Development, Sebela Women’s Health. “This distinction further supports our belief that the novel design of MIUDELLA will offer an innovative option for birth control for women nationwide.”
Guidelines from the American College of Obstetrics and Gynecology state that long-acting reversible contraceptive (LARC) methods, including intrauterine devices and contraceptive implants, are the most effective contraceptive methods, have few contraindications, and are appropriate for almost all patients.1 While there are a variety of contraceptive methods available to women, 41.6 percent of pregnancies in the U.S. are unintended.2
INDICATION FOR MIUDELLA®
MIUDELLA® is a copper-containing intrauterine system (IUS) indicated for prevention of pregnancy in females of reproductive potential for up to 3 years.IMPORTANT SAFETY INFORMATION
- WARNING: Improper insertion of intrauterine systems, including MIUDELLA®, increases the risk of complications.
- Proper training prior to first use of MIUDELLA® can minimize the risk of improper insertion.
- MIUDELLA® is available only through a restricted program under a Risk Evaluation and Mitigation Strategy (REMS) called the MIUDELLA® REMS program to ensure healthcare providers are trained on the proper insertion of MIUDELLA® prior to first use. Further information is available at miudellarems.com and 1-855-337-0772.
- Contraindications: Don’t use MIUDELLA® if you are or may be pregnant, have a uterine anomaly that may affect correct placement, acute pelvic inflammatory disease, postpartum endometritis or postabortal endometritis in past 3 months, known or suspected uterine or cervical malignancy, for use as post-coital contraception (emergency contraception), unexplained bleeding, untreated acute cervicitis or vaginitis or other lower genital tract infection, conditions associated with increased susceptibility to pelvic infections, Wilson’s disease, a previously placed IUS that has not been removed and/or hypersensitivity to any component of MIUDELLA® including copper, nitinol or any trace elements present in the copper components of MIUDELLA®.
- Pregnancy with MIUDELLA® is rare but can be life threatening and cause infertility or loss of pregnancy.
- MIUDELLA® may attach to or go through the uterus and cause other problems.
- Tell your healthcare provider (HCP) if you develop severe pain or fever shortly after placement, miss a period, have abdominal pain, or if MIUDELLA® comes out. If it comes out, use backup birth control.
- At first, periods may be altered and result in heavier and longer bleeding with spotting in between.
- Tell your HCP you have MIUDELLA® before having an MRI or a medical procedure using heat therapy.
- Additional common side effects include painful periods, pelvic discomfort/pain, procedural pain, post procedural bleeding, and pain during sex.
- MIUDELLA® does not protect against HIV or STDs.
Only you and your HCP can decide if MIUDELLA® is right for you. Available by prescription only. For additional information or to report suspected adverse reactions, please contact Sebela Women’s Health Inc. at 1-866-246-2133. You are encouraged to report negative side effects of prescription drugs to the FDA at www.fda.gov/medwatch or call 1-800-FDA-1088.
Click here for the Full Prescribing Information for MIUDELLA®.
About Sebela Pharmaceuticals®
Sebela Pharmaceuticals is a US pharmaceutical company with a market leading position in gastroenterology and a focus on innovation in women’s health. In addition to MIUDELLA®, Sebela Women’s Health has another next-generation hormonal IUD for contraception in late-stage clinical development. Braintree Laboratories, Inc., a part of Sebela Pharmaceuticals, is the market leader in colonoscopy screening preparations for over 35 years, having invented, developed and commercialized a broad portfolio of innovative prescription colonoscopy preparations and multiple gastroenterology products. Braintree also has several gastroenterology programs in late-stage clinical development including Tegoprazan which is in phase 3 trials for gastro-esophageal reflux disease (GERD), specifically, erosive esophagitis (EE) and non-erosive reflux disease (NERD).
Sebela Pharmaceuticals has offices/operations in Roswell, GA; Braintree, MA; and Dublin, Ireland. Please visit sebelapharma.com for more information or call 844-732-3521.
MIUDELLA is a registered trademark of Sebela Women’s Health Inc.
Forward Looking Statements
This press release and any statements made for and during any presentation or meeting contain forward- looking statements related to Sebela Women’s Health Inc. under the safe harbor provisions of Section 21E of the Private Securities Litigation Reform Act of 1995, as amended, and are subject to risks and uncertainties that could cause actual results to differ materially from those projected. In some cases, forward-looking statements can be identified by terminology such as “will,” “may,” “should,” “could,” “expects,” “intends,” “plans,” “aims,” “anticipates,” “believes,” “estimates,” “predicts,” “potential,” “continue,” or the negative of these terms or other comparable terminology, although not all forward-looking statements contain these words. There are a number of factors that could cause actual events to differ materially from those indicated by such forward-looking statements. These factors include, but are not limited to, the development, launch, introduction and commercial potential of IUDs as described herein; growth and opportunity, including peak sales and the potential demand for these IUDs, as well as their potential impact on applicable markets; market size; substantial competition; our ability to continue as a growing concern; our need for additional financing; uncertainties of patent protection and litigation; uncertainties of government or third-party payer reimbursement; dependence upon third parties supply and manufacturing uncertainties; our financial performance and results, including the risk that we are unable to manage our operating expenses or cash use for operations, or are unable to commercialize our products, within the guided ranges or otherwise as expected; and risks related to failure to obtain FDA clearances or approvals and noncompliance with FDA regulations. As with any pharmaceutical under development, there are significant risks in the development, regulatory approval and commercialization of new products. While the list of factors presented here is considered representative, no such list should be considered to be a complete statement of all potential risks and uncertainties. Unlisted factors may present significant additional obstacles to the realization of forward-looking statements. Forward-looking statements included herein are made as of the date hereof, are based on current expectations, and Sebela Women’s Health Inc. does not undertake any obligation to update publicly such statements to reflect subsequent events or circumstances except as required by law.
Contact
Sebela Women’s Health
Erinn White
[email protected]
917-769-27851 ACOG, Clinical Practice Bulletin #186, Nov. 2017 reaffirmed 2021; Committee Statement #5, April 2023. Accessed on April 18, 2023: https://www.acog.org/clinical/clinical-guidance/practice-bulletin/articles/2017/11/long-acting-reversible-contraception-implants-and-intrauterine-devices and https://www.acog.org/clinical/clinical-guidance/committee-statement/articles/2023/03/increasing-access-to-intrauterine-devices-and-contraceptive-implants
2 Centers for Disease Control and Prevention. Accessed on Feb. 18, 2025. https://www.cdc.gov/reproductive-health/hcp/unintended-pregnancy/index.html#:~:text=Overview,2010%20to%2035.7%20in%202019SOURCE Sebela Pharmaceuticals Inc
Continue Reading