Cooke GS, Flower B, Cunningham E, et al. Progress towards elimination of viral hepatitis: a lancet gastroenterology & hepatology commission update. Lancet Gastroenterol Hepatol. 2024;9(4):346–65.
Google Scholar
Terrault NA, Levy MT, Cheung KW, Jourdain G. Viral hepatitis and pregnancy. Nat Reviews Gastroenterol Hepatol. 2021;18(2):117–30.
WHO. Global health sector strategy on viral hepatitis 2016–2021. Towards ending viral hepatitis. 2016; https://www.who.int/publications/i/item/WHO-HIV-2016.06
Odenwald MA, Paul S. Viral hepatitis: past, present, and future. World J Gastroenterol. 2022;28(14):1405–29.
Google Scholar
Castaneda D, Gonzalez AJ, Alomari M, Tandon K, Zervos XB. From hepatitis A to E: A critical review of viral hepatitis. World J Gastroenterol. 2021;27(16):1691–715.
Google Scholar
Fitzmaurice C, Allen C, Barber RM, et al. Global, regional, and National cancer incidence, mortality, years of life lost, years lived with disability, and Disability-Adjusted life-years for 32 cancer groups, 1990 to 2015: A systematic analysis for the global burden of disease study. JAMA Oncol. 2017;3(4):524–48.
Google Scholar
Wen B, Te L, Bai C, et al. Relative contribution of hepatitis B and C viruses in primary liver cancer in china: A systematic review and meta-analysis. J Infect. 2024;89(6):106298.
Google Scholar
Chan SL, Wong VW, Qin S, Chan HL. Infection and cancer: the case of hepatitis B. J Clin Oncology: Official J Am Soc Clin Oncol. 2016;34(1):83–90.
Bogliotti Y, Vander Roest M, Mattis AN, et al. Clinical application of induced Hepatocyte-like cells produced from mesenchymal stromal cells: A literature review. Cells. 2022;11:13.
Hamburg-Shields E, Prasad M. Infectious hepatitis in pregnancy. Clin Obstet Gynecol. 2020;63(1):175–92.
Google Scholar
WHO, Hepatitis B. 2024; https://www.who.int/news-room/fact-sheets/detail/hepatitis-b
Global prevalence. treatment, and prevention of hepatitis B virus infection in 2016: a modelling study. Lancet Gastroenterol Hepatol. 2018;3(6):383–403.
WHO. Guidelines for the prevention, diagnosis, care and treatment for people with chronic hepatitis B infection. 2024; https://www.who.int/publications/i/item/9789240090903
McMahon BJ. The natural history of chronic hepatitis B virus infection. Semin Liver Dis. 2004;24(Suppl 1):17–21.
Google Scholar
Veronese P, Dodi I, Esposito S, Indolfi G. Prevention of vertical transmission of hepatitis B virus infection. World J Gastroenterol. 2021;27(26):4182–93.
Google Scholar
Indolfi G, Easterbrook P, Dusheiko G, et al. Hepatitis B virus infection in children and adolescents. Lancet Gastroenterol Hepatol. 2019;4(6):466–76.
Google Scholar
Lu H, Cao W, Zhang L, et al. Effects of hepatitis B virus infection and strategies for preventing mother-to-child transmission on maternal and fetal T-cell immunity. Front Immunol. 2023;14:1122048.
Google Scholar
Iannacone M, Guidotti LG. Immunobiology and pathogenesis of hepatitis B virus infection. Nat Rev Immunol. 2022;22(1):19–32.
Google Scholar
Hillis WD. VIRAL HEPATITIS ASSOCIATED WITH SUB-HUMAN PRIMATES. Transfusion. 1963;3:445–54.
Google Scholar
Walter E, Keist R, Niederöst B, Pult I, Blum HE. Hepatitis B virus infection of Tupaia hepatocytes in vitro and in vivo. Hepatology (Baltimore MD). 1996;24(1):1–5.
Google Scholar
Li J, Shi TD, Han JF, et al. A systematic study of Tupaia as a model for human acute hepatitis B infection. J Vet Med Sci. 2021;83(6):1004–11.
Google Scholar
Haering C, McMahon B, Harris A, et al. Hepatitis B virus elimination status and strategies in circumpolar countries, 2020. Int J Circumpolar Health. 2021;80(1):1986975.
Google Scholar
Zheng JR, Wang ZL, Feng B. Hepatitis B functional cure and immune response. Front Immunol. 2022;13:1075916.
Google Scholar
Wong GLH, Gane E, Lok ASF. How to achieve functional cure of HBV: stopping nucs, adding interferon or new drug development? J Hepatol. 2022;76(6):1249–62.
Google Scholar
Jeng WJ, Lok ASF. What will it take to cure hepatitis B? Hepatol Commun 2023;7(4).
Lok AS, Zoulim F, Dusheiko G, Ghany MG. Hepatitis B cure: from discovery to regulatory approval. Hepatology (Baltimore MD). 2017;66(4):1296–313.
Google Scholar
Nassal M. HBV cccdna: viral persistence reservoir and key obstacle for a cure of chronic hepatitis B. Gut. 2015;64(12):1972–84.
Google Scholar
Khanam A, Chua JV, Kottilil S. Immunopathology of chronic hepatitis B infection: role of innate and adaptive immune response in disease progression. Int J Mol Sci 2021;22(11).
Peeridogaheh H, Meshkat Z, Habibzadeh S, et al. Current concepts on Immunopathogenesis of hepatitis B virus infection. Virus Res. 2018;245:29–43.
Google Scholar
Zhang L, Zhang F, Ma Z, Jin J. Hepatitis B virus infection, infertility, and assisted reproduction. J Zhejiang Univ Sci B. 2024;25(8):672–85.
Google Scholar
Thimme R, Bertoletti A, Iannacone M. Beyond exhaustion: the unique characteristics of CD8(+) T cell dysfunction in chronic HBV infection. Nat Rev Immunol. 2024;24(11):775–6.
Google Scholar
Panduro A, Roman S, Laguna-Meraz S, Jose-Abrego A, Hepatitis B, Virus Genotype H. Epidemiological, molecular, and clinical characteristics in Mexico. Viruses 2023;15(11).
Dong Z, Li JR, Zhao ZX, et al. Molecular epidemiology of hepatitis B virus genotypes and subgenotypes in ethnic minority populations, Yunnan province, China. Epidemiol Infect. 2021;150:e11.
Google Scholar
Sarrazin S, Lamanna WC, Esko JD. Heparan sulfate proteoglycans. Cold Spring Harb Perspect Biol 2011;3(7).
Schulze A, Gripon P, Urban S. Hepatitis B virus infection initiates with a large surface protein-dependent binding to Heparan sulfate proteoglycans. Hepatology (Baltimore MD). 2007;46(6):1759–68.
Google Scholar
Leistner CM, Gruen-Bernhard S, Glebe D. Role of glycosaminoglycans for binding and infection of hepatitis B virus. Cell Microbiol. 2008;10(1):122–33.
Google Scholar
Herrscher C, Roingeard P, Blanchard E. Hepatitis B virus entry into cells. Cells 2020;9(6).
Verrier ER, Colpitts CC, Bach C, et al. A targeted functional RNA interference screen uncovers glypican 5 as an entry factor for hepatitis B and D viruses. Hepatology (Baltimore MD). 2016;63(1):35–48.
Google Scholar
Yan H, Zhong G, Xu G et al. Sodium taurocholate cotransporting polypeptide is a functional receptor for human hepatitis B and D virus. eLife 2012;3.
Asami J, Kimura KT, Fujita-Fujiharu Y, et al. Structure of the bile acid transporter and HBV receptor NTCP. Nature. 2022;606(7916):1021–6.
Google Scholar
Zhang Z, Zhang Q, Zhang Y, et al. Role of sodium taurocholate cotransporting polypeptide (NTCP) in HBV-induced hepatitis: opportunities for developing novel therapeutics. Biochem Pharmacol. 2024;219:115956.
Google Scholar
Döring B, Lütteke T, Geyer J, Petzinger E. The SLC10 carrier family: transport functions and molecular structure. Curr Top Membr. 2012;70:105–68.
Google Scholar
Wang J, Qu B, Zhang F, et al. Stem Cell-Derived Hepatocyte-Like cells as model for viral hepatitis research. Stem Cells Int. 2019;2019:9605252.
Google Scholar
Iwamoto M, Saso W, Sugiyama R, et al. Epidermal growth factor receptor is a host-entry cofactor triggering hepatitis B virus internalization. Proc Natl Acad Sci USA. 2019;116(17):8487–92.
Google Scholar
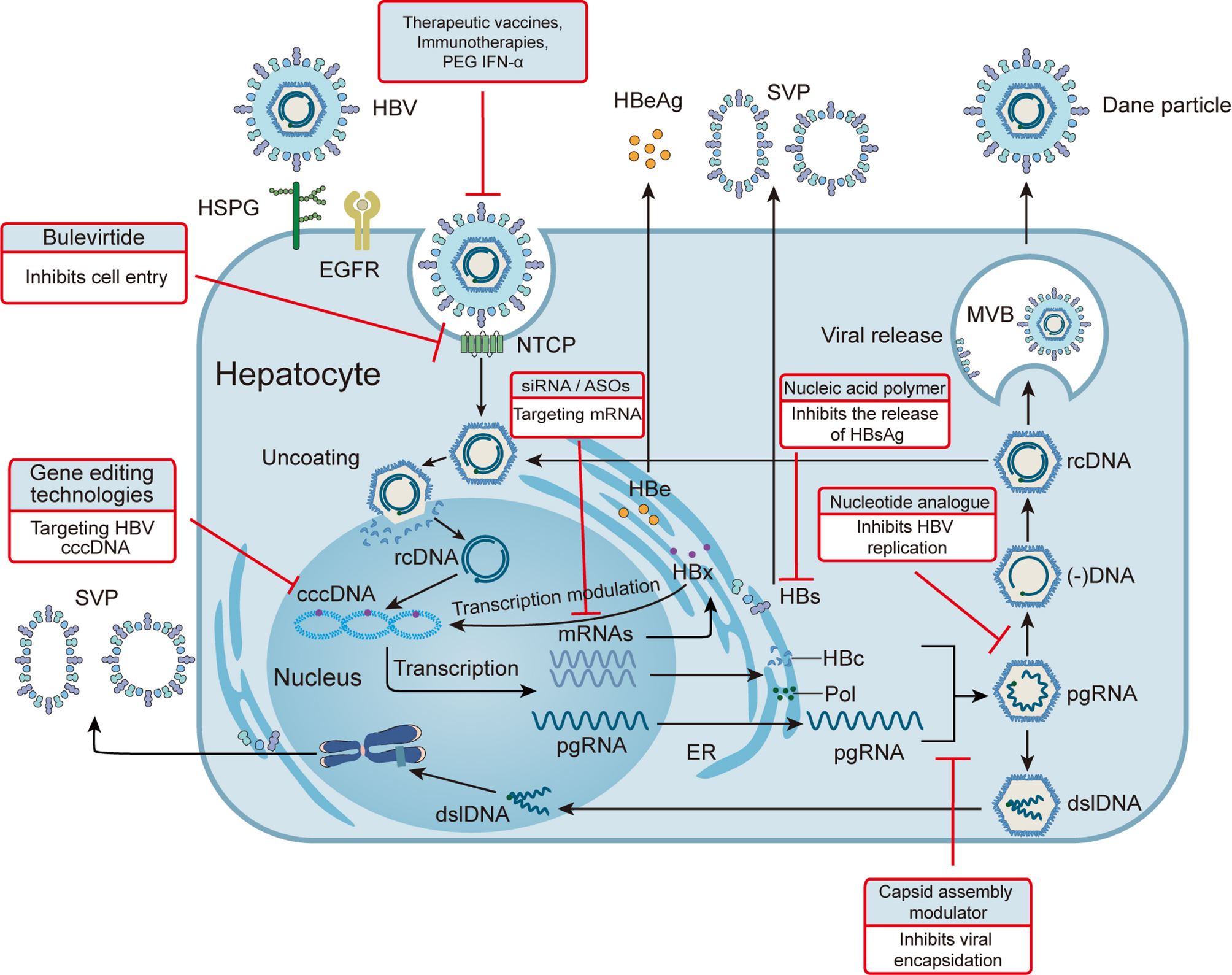
Xia Y, Guo H. Hepatitis B virus cccdna: formation, regulation and therapeutic potential. Antiviral Res. 2020;180:104824.
Google Scholar
Raimondo G, Locarnini S, Pollicino T, Levrero M, Zoulim F, Lok AS. Update of the statements on biology and clinical impact of occult hepatitis B virus infection. J Hepatol. 2019;71(2):397–408.
Google Scholar
Martinez MG, Boyd A, Combe E, Testoni B, Zoulim F. Covalently closed circular DNA: the ultimate therapeutic target for curing HBV infections. J Hepatol. 2021;75(3):706–17.
Google Scholar
Jeng WJ, Papatheodoridis GV, Lok ASF, Hepatitis B. Lancet (London England). 2023;401(10381):1039–52.
Google Scholar
Yan W, Rao D, Fan F, Liang H, Zhang Z, Dong H. Hepatitis B virus X protein and TGF-β: partners in the carcinogenic journey of hepatocellular carcinoma. Front Oncol. 2024;14:1407434.
Google Scholar
Tsukuda S, Watashi K. Hepatitis B virus biology and life cycle. Antiviral Res. 2020;182:104925.
Google Scholar
Hu J, Liu K. Complete and incomplete hepatitis B virus particles: formation, function, and application. Viruses 2017;9(3).
Brakenhoff SM, de Knegt RJ, van Campenhout MJH et al. End-of-treatment HBsAg, HBcrAg and HBV RNA predict the risk of off-treatment ALT flares in chronic hepatitis B patients. Journal of microbiology, immunology, and infection = Wei mian yu gan ran za zhi. 2023;56(1):31–39.
Zoulim F, Carosi G, Greenbloom S, et al. Quantification of HBsAg in nucleos(t)ide-naïve patients treated for chronic hepatitis B with Entecavir with or without Tenofovir in the BE-LOW study. J Hepatol. 2015;62(1):56–63.
Google Scholar
Brunetto MR. A new role for an old marker, HBsAg. J Hepatol. 2010;52(4):475–7.
Google Scholar
Sonneveld MJ, Chiu SM, Park JY, et al. Lower pretreatment HBV DNA levels are associated with better off-treatment outcomes after nucleo(s)tide analogue withdrawal in patients with HBeAg-negative chronic hepatitis B: A multicentre cohort study. JHEP Reports: Innov Hepatol. 2023;5(8):100790.
Qiu Y, Tang Q, Liu XQ, Xue YL, Zeng Y, Hu P. Hepatitis B core-related antigen as a promising serological marker for monitoring hepatitis B virus cure. World J Hepatol. 2025;17(1):98658.
Google Scholar
Liu Y, Jiang M, Xue J, Yan H, Liang X. Serum HBV RNA quantification: useful for monitoring natural history of chronic hepatitis B infection. BMC Gastroenterol. 2019;19(1):53.
Google Scholar
Lu F, Wang J, Chen X, Xu D, Xia N. Potential use of serum HBV RNA in antiviral therapy for chronic hepatitis B in the era of nucleos(t)ide analogs. Front Med. 2017;11(4):502–8.
Google Scholar
Mak LY, Boettler T, Gill US. HBV biomarkers and their role in guiding treatment decisions. Semin Liver Dis. 2024;44(4):474–91.
Google Scholar
Busch K, Thimme R. Natural history of chronic hepatitis B virus infection. Med Microbiol Immunol. 2015;204(1):5–10.
Google Scholar
Mazzaro C, Adinolfi LE, Pozzato G et al. Extrahepatic Manifestations of Chronic HBV Infection and the Role of Antiviral Therapy. Journal of clinical medicine. 2022;11(21).
Cacoub P, Asselah T, Hepatitis B. Virus infection and Extra-Hepatic manifestations: A systemic disease. Am J Gastroenterol. 2022;117(2):253–63.
Google Scholar
Baig S, Alamgir M. The extrahepatic manifestations of hepatitis B virus. J Coll Physicians Surg Pak. 2008;18(7):451–7.
Google Scholar
Global change in. Hepatitis C virus prevalence and cascade of care between 2015 and 2020: a modelling study. Lancet Gastroenterol Hepatol. 2022;7(5):396–415.
WHO, Hepatitis C. 2024; https://www.who.int/news-room/fact-sheets/detail/hepatitis-c
Martinello M, Solomon SS, Terrault NA, Dore GJ, Hepatitis C. Lancet (London England). 2023;402(10407):1085–96.
Google Scholar
Morozov VA, Lagaye S. Hepatitis C virus: morphogenesis, infection and therapy. World J Hepatol. 2018;10(2):186–212.
Google Scholar
Midgard H, Weir A, Palmateer N, et al. HCV epidemiology in high-risk groups and the risk of reinfection. J Hepatol. 2016;65(1 Suppl):S33–45.
Google Scholar
Graham CS, Swan T. A path to eradication of hepatitis C in low- and middle-income countries. Antiviral Res. 2015;119:89–96.
Google Scholar
Smith DB, Bukh J, Kuiken C, et al. Expanded classification of hepatitis C virus into 7 genotypes and 67 subtypes: updated criteria and genotype assignment web resource. Hepatology (Baltimore MD). 2014;59(1):318–27.
Google Scholar
Pawlotsky JM, Tsakiris L, Roudot-Thoraval F, et al. Relationship between hepatitis C virus genotypes and sources of infection in patients with chronic hepatitis C. J Infect Dis. 1995;171(6):1607–10.
Google Scholar
Zein NN. Clinical significance of hepatitis C virus genotypes. Clin Microbiol Rev. 2000;13(2):223–35.
Google Scholar
Sallam M, Khalil R. Contemporary insights into hepatitis C virus: A comprehensive review. Microorganisms 2024;12(6).
Martinez MA, Franco S. Therapy implications of hepatitis C virus genetic diversity. Viruses 2020;13(1).
Tsukiyama-Kohara K, Kohara M, Hepatitis C, Virus. Viral quasispecies and genotypes. Int J Mol Sci 2017;19(1).
Manns MP, Buti M, Gane E, et al. Hepatitis C virus infection. Nat Reviews Disease Primers. 2017;3:17006.
Google Scholar
Manns MP, Maasoumy B. Breakthroughs in hepatitis C research: from discovery to cure. Nat Reviews Gastroenterol Hepatol. 2022;19(8):533–50.
Bukh J. The history of hepatitis C virus (HCV): basic research reveals unique features in phylogeny, evolution and the viral life cycle with new perspectives for epidemic control. J Hepatol. 2016;65(1 Suppl):S2–21.
Google Scholar
Adams RL, Pirakitikulr N, Pyle AM. Functional RNA structures throughout the hepatitis C virus genome. Curr Opin Virol. 2017;24:79–86.
Google Scholar
Méndez-Sánchez N, Coronel-Castillo CE, Ramírez-Mejía MM. Chronic hepatitis C virus infection, extrahepatic disease and the impact of new Direct-Acting antivirals. Pathogens (Basel Switzerland) 2024;13(4).
Merz A, Long G, Hiet MS, et al. Biochemical and morphological properties of hepatitis C virus particles and determination of their lipidome. J Biol Chem. 2011;286(4):3018–32.
Google Scholar
Lindenbach BD, Meuleman P, Ploss A, et al. Cell culture-grown hepatitis C virus is infectious in vivo and can be recultured in vitro. Proc Natl Acad Sci USA. 2006;103(10):3805–9.
Google Scholar
Alazard-Dany N, Denolly S, Boson B, Cosset FL. Overview of HCV life cycle with a special focus on current and possible future antiviral targets. Viruses 2019;11(1).
Pileri P, Uematsu Y, Campagnoli S, et al. Binding of hepatitis C virus to CD81. Sci (New York NY). 1998;282(5390):938–41.
Scarselli E, Ansuini H, Cerino R, et al. The human scavenger receptor class B type I is a novel candidate receptor for the hepatitis C virus. EMBO J. 2002;21(19):5017–25.
Google Scholar
Evans MJ, von Hahn T, Tscherne DM, et al. Claudin-1 is a hepatitis C virus co-receptor required for a late step in entry. Nature. 2007;446(7137):801–5.
Google Scholar
Ploss A, Evans MJ, Gaysinskaya VA, et al. Human occludin is a hepatitis C virus entry factor required for infection of mouse cells. Nature. 2009;457(7231):882–6.
Google Scholar
Agnello V, Abel G, Elfahal M, Knight GB, Zhang QX. Hepatitis C virus and other flaviviridae viruses enter cells via low density lipoprotein receptor. Proc Natl Acad Sci USA. 1999;96(22):12766–71.
Google Scholar
Lupberger J, Zeisel MB, Xiao F, et al. EGFR and EphA2 are host factors for hepatitis C virus entry and possible targets for antiviral therapy. Nat Med. 2011;17(5):589–95.
Google Scholar
Sainz B Jr., Barretto N, Martin DN, et al. Identification of the Niemann-Pick C1-like 1 cholesterol absorption receptor as a new hepatitis C virus entry factor. Nat Med. 2012;18(2):281–5.
Google Scholar
Sharma NR, Mateu G, Dreux M, Grakoui A, Cosset FL, Melikyan GB. Hepatitis C virus is primed by CD81 protein for low pH-dependent fusion. J Biol Chem. 2011;286(35):30361–76.
Google Scholar
Choo QL, Kuo G, Weiner AJ, Overby LR, Bradley DW, Houghton M. Isolation of a cDNA clone derived from a blood-borne non-A, non-B viral hepatitis genome. Sci (New York NY). 1989;244(4902):359–62.
Niepmann M. Hepatitis C virus RNA translation. Curr Top Microbiol Immunol. 2013;369:143–66.
Google Scholar
Honda M, Beard MR, Ping LH, Lemon SM. A phylogenetically conserved stem-loop structure at the 5’ border of the internal ribosome entry site of hepatitis C virus is required for cap-independent viral translation. J Virol. 1999;73(2):1165–74.
Google Scholar
Hoffman B, Liu Q. Hepatitis C viral protein translation: mechanisms and implications in developing antivirals. Liver International: Official J Int Association Study Liver. 2011;31(10):1449–67.
Scheel TK, Rice CM. Understanding the hepatitis C virus life cycle paves the way for highly effective therapies. Nat Med. 2013;19(7):837–49.
Google Scholar
Bartenschlager R, Lohmann V, Wilkinson T, Koch JO. Complex formation between the NS3 serine-type proteinase of the hepatitis C virus and NS4A and its importance for polyprotein maturation. J Virol. 1995;69(12):7519–28.
Google Scholar
Dubuisson J, Cosset FL. Virology and cell biology of the hepatitis C virus life cycle: an update. J Hepatol. 2014;61(1 Suppl):S3–13.
Google Scholar
Foster TL, Belyaeva T, Stonehouse NJ, Pearson AR, Harris M. All three domains of the hepatitis C virus nonstructural NS5A protein contribute to RNA binding. J Virol. 2010;84(18):9267–77.
Google Scholar
Appel N, Zayas M, Miller S, et al. Essential role of domain III of nonstructural protein 5A for hepatitis C virus infectious particle assembly. PLoS Pathog. 2008;4(3):e1000035.
Google Scholar
Tellinghuisen TL, Foss KL, Treadaway J. Regulation of hepatitis C virion production via phosphorylation of the NS5A protein. PLoS Pathog. 2008;4(3):e1000032.
Google Scholar
Gu M, Rice CM. Three conformational snapshots of the hepatitis C virus NS3 helicase reveal a ratchet translocation mechanism. Proc Natl Acad Sci USA. 2010;107(2):521–8.
Google Scholar
Gouttenoire J, Penin F, Moradpour D. Hepatitis C virus nonstructural protein 4B: a journey into unexplored territory. Rev Med Virol. 2010;20(2):117–29.
Google Scholar
Wozniak AL, Griffin S, Rowlands D, et al. Intracellular proton conductance of the hepatitis C virus p7 protein and its contribution to infectious virus production. PLoS Pathog. 2010;6(9):e1001087.
Google Scholar
Herker E, Harris C, Hernandez C, et al. Efficient hepatitis C virus particle formation requires Diacylglycerol acyltransferase-1. Nat Med. 2010;16(11):1295–8.
Google Scholar
Ariumi Y, Kuroki M, Maki M, et al. The ESCRT system is required for hepatitis C virus production. PLoS ONE. 2011;6(1):e14517.
Google Scholar
Barouch-Bentov R, Neveu G, Xiao F et al. Hepatitis C virus proteins interact with the endosomal sorting complex required for transport (ESCRT) machinery via ubiquitination to facilitate viral envelopment. mBio 2016;7(6).
Falcón V, Acosta-Rivero N, González S, et al. Ultrastructural and biochemical basis for hepatitis C virus morphogenesis. Virus Genes. 2017;53(2):151–64.
Google Scholar
Mankouri J, Walter C, Stewart H, et al. Release of infectious hepatitis C virus from Huh7 cells occurs via a trans-Golgi Network-to-Endosome pathway independent of Very-Low-Density lipoprotein secretion. J Virol. 2016;90(16):7159–70.
Google Scholar
Baber AS, Suganthan B, Ramasamy RP. Current advances in hepatitis C diagnostics. J Biol Eng. 2024;18(1):48.
Google Scholar
Westbrook RH, Dusheiko G. Natural history of hepatitis C. J Hepatol. 2014;61(1 Suppl):S58–68.
Google Scholar
Bowen DG, Walker CM. Adaptive immune responses in acute and chronic hepatitis C virus infection. Nature. 2005;436(7053):946–52.
Google Scholar
Santantonio T, Wiegand J, Gerlach JT. Acute hepatitis C: current status and remaining challenges. J Hepatol. 2008;49(4):625–33.
Google Scholar
Liu CH, Kao JH. Acute hepatitis C virus infection: clinical update and remaining challenges. Clin Mol Hepatol. 2023;29(3):623–42.
Google Scholar
Takano S, Omata M, Ohto M, Satomura Y. Prospective assessment of incidence of fulminant hepatitis in post-transfusion hepatitis: a study of 504 cases. Dig Dis Sci. 1994;39(1):28–32.
Google Scholar
Kaplan DE, Hepatitis C, Virus. Ann Intern Med. 2020;173(5):Itc33–48.
Google Scholar
Negro F. Natural history of hepatic and extrahepatic hepatitis C virus diseases and impact of Interferon-Free HCV therapy. Cold Spring Harbor Perspect Med 2020;10(4).
Shen C, Jiang X, Li M, Luo Y. Hepatitis virus and hepatocellular carcinoma: recent advances. Cancers 2023;15(2).
Axley P, Ahmed Z, Ravi S, Singal AK, Hepatitis C. Virus and hepatocellular carcinoma: A narrative review. J Clin Translational Hepatol. 2018;6(1):79–84.
Khullar V, Firpi RJ. Hepatitis C cirrhosis: new perspectives for diagnosis and treatment. World J Hepatol. 2015;7(14):1843–55.
Google Scholar
Maqsood Q, Sumrin A, Iqbal M, et al. Hepatitis C virus/hepatitis B virus coinfection: current prospectives. Antivir Ther. 2023;28(4):13596535231189643.
Google Scholar
Wangensteen KJ, Chang KM. Multiple roles for hepatitis B and C viruses and the host in the development of hepatocellular carcinoma. Hepatology (Baltimore MD). 2021;73(1Suppl 1):27–37.
Google Scholar
Péneau C, Imbeaud S, La Bella T, et al. Hepatitis B virus integrations promote local and distant oncogenic driver alterations in hepatocellular carcinoma. Gut. 2022;71(3):616–26.
Google Scholar
Jia L, Gao Y, He Y, Hooper JD, Yang P. HBV induced hepatocellular carcinoma and related potential immunotherapy. Pharmacol Res. 2020;159:104992.
Google Scholar
Yuan H, Xu R, Li S, et al. The malignant transformation of viral hepatitis to hepatocellular carcinoma: mechanisms and interventions. MedComm. 2025;6(3):e70121.
Google Scholar
Heredia-Torres TG, Rincón-Sánchez AR, Lozano-Sepúlveda SA et al. Unraveling the molecular mechanisms involved in HCV-Induced carcinogenesis. Viruses 2022;14(12).
Rizzetto M, Canese MG, Aricò S, et al. Immunofluorescence detection of new antigen-antibody system (delta/anti-delta) associated to hepatitis B virus in liver and in serum of HBsAg carriers. Gut. 1977;18(12):997–1003.
Google Scholar
Koh C, Heller T, Glenn JS. Pathogenesis of and new therapies for hepatitis D. Gastroenterology. 2019;156(2):461–e476461.
Google Scholar
Farci P, Niro GA. Clinical features of hepatitis D. Semin Liver Dis. 2012;32(3):228–36.
Google Scholar
Pearlman B. Hepatitis delta infection: A clinical review. Semin Liver Dis. 2023;43(3):293–304.
Google Scholar
Negro F, Lok AS. Hepatitis D: A review. JAMA. 2023;330(24):2376–87.
Google Scholar
Miao Z, Xie Z, Ren L, Pan Q. Hepatitis D: advances and challenges. Chin Med J (Engl). 2022;135(7):767–73.
Google Scholar
Wranke A, Pinheiro Borzacov LM, Parana R, et al. Clinical and virological heterogeneity of hepatitis delta in different regions world-wide: the hepatitis delta international network (HDIN). Liver International: Official J Int Association Study Liver. 2018;38(5):842–50.
Stockdale AJ, Kreuels B, Henrion MYR, et al. The global prevalence of hepatitis D virus infection: systematic review and meta-analysis. J Hepatol. 2020;73(3):523–32.
Google Scholar
Asselah T, Loureiro D, Tout I, et al. Future treatments for hepatitis delta virus infection. Liver International: Official J Int Association Study Liver. 2020;40(Suppl 1):54–60.
Niro GA, Ferro A, Cicerchia F, Brascugli I, Durazzo M. Hepatitis delta virus: from infection to new therapeutic strategies. World J Gastroenterol. 2021;27(24):3530–42.
Google Scholar
Zhang Z, Urban S. New insights into HDV persistence: the role of interferon response and implications for upcoming novel therapies. J Hepatol. 2021;74(3):686–99.
Google Scholar
Taylor JM. Infection by Hepatitis Delta Virus. Viruses. 2020;12(6).
Griffin BL, Chasovskikh S, Dritschilo A, Casey JL. Hepatitis delta antigen requires a flexible quasi-double-stranded RNA structure to bind and condense hepatitis delta virus RNA in a ribonucleoprotein complex. J Virol. 2014;88(13):7402–11.
Google Scholar
Defenbaugh DA, Johnson M, Chen R, Zheng YY, Casey JL. Hepatitis delta antigen requires a minimum length of the hepatitis delta virus unbranched rod RNA structure for binding. J Virol. 2009;83(9):4548–56.
Google Scholar
Khalfi P, Kennedy PT, Majzoub K, Asselah T. Hepatitis D virus: improving virological knowledge to develop new treatments. Antiviral Res. 2023;209:105461.
Google Scholar
Caviglia GP, Ciancio A, Rizzetto M. A review of HDV infection. Viruses 2022;14(8).
Sureau C, Negro F. The hepatitis delta virus: replication and pathogenesis. J Hepatol. 2016;64(1 Suppl):S102–16.
Google Scholar
Loureiro D, Castelnau C, Tout I, et al. New therapies for hepatitis delta virus infection. Liver International: Official J Int Association Study Liver. 2021;41(Suppl 1):30–7.
Lamas Longarela O, Schmidt TT, Schöneweis K, et al. Proteoglycans act as cellular hepatitis delta virus attachment receptors. PLoS ONE. 2013;8(3):e58340.
Google Scholar
Ni Y, Lempp FA, Mehrle S, et al. Hepatitis B and D viruses exploit sodium taurocholate co-transporting polypeptide for species-specific entry into hepatocytes. Gastroenterology. 2014;146(4):1070–83.
Google Scholar
Macnaughton TB, Shi ST, Modahl LE, Lai MM. Rolling circle replication of hepatitis delta virus RNA is carried out by two different cellular RNA polymerases. J Virol. 2002;76(8):3920–7.
Google Scholar
Greco-Stewart VS, Schissel E, Pelchat M. The hepatitis delta virus RNA genome interacts with the human RNA polymerases I and III. Virology. 2009;386(1):12–5.
Google Scholar
Hsieh SY, Chao M, Coates L, Taylor J. Hepatitis delta virus genome replication: a polyadenylated mRNA for delta antigen. J Virol. 1990;64(7):3192–8.
Google Scholar
Mentha N, Clément S, Negro F, Alfaiate D. A review on hepatitis D: from virology to new therapies. J Adv Res. 2019;17:3–15.
Google Scholar
Casey JL. Control of ADAR1 editing of hepatitis delta virus RNAs. Curr Top Microbiol Immunol. 2012;353:123–43.
Google Scholar
Lempp FA, Ni Y, Urban S. Hepatitis delta virus: insights into a peculiar pathogen and novel treatment options. Nat Reviews Gastroenterol Hepatol. 2016;13(10):580–9.
Urban S, Neumann-Haefelin C, Lampertico P. Hepatitis D virus in 2021: virology, immunology and new treatment approaches for a difficult-to-treat disease. Gut. 2021;70(9):1782–94.
Google Scholar
Modahl LE, Lai MM. The large delta antigen of hepatitis delta virus potently inhibits genomic but not antigenomic RNA synthesis: a mechanism enabling initiation of viral replication. J Virol. 2000;74(16):7375–80.
Google Scholar
Hong SY, Chen PJ. Phosphorylation of Serine 177 of the small hepatitis delta antigen regulates viral antigenomic RNA replication by interacting with the processive RNA polymerase II. J Virol. 2010;84(3):1430–8.
Google Scholar
Glenn JS, Watson JA, Havel CM, White JM. Identification of a prenylation site in delta virus large antigen. Sci (New York NY). 1992;256(5061):1331–3.
Hwang SB, Lai MM. Isoprenylation mediates direct protein-protein interactions between hepatitis large delta antigen and hepatitis B virus surface antigen. J Virol. 1993;67(12):7659–62.
Google Scholar
Bordier BB, Marion PL, Ohashi K, et al. A prenylation inhibitor prevents production of infectious hepatitis delta virus particles. J Virol. 2002;76(20):10465–72.
Google Scholar
Bordier BB, Ohkanda J, Liu P, et al. In vivo antiviral efficacy of prenylation inhibitors against hepatitis delta virus. J Clin Investig. 2003;112(3):407–14.
Google Scholar
Bonino F, Heermann KH, Rizzetto M, Gerlich WH. Hepatitis delta virus: protein composition of delta antigen and its hepatitis B virus-derived envelope. J Virol. 1986;58(3):945–50.
Google Scholar
Weiner AJ, Choo QL, Wang KS, et al. A single antigenomic open reading frame of the hepatitis delta virus encodes the epitope(s) of both hepatitis delta antigen polypeptides p24 delta and p27 delta. J Virol. 1988;62(2):594–9.
Google Scholar
Watanabe T, Sorensen EM, Naito A, Schott M, Kim S, Ahlquist P. Involvement of host cellular multivesicular body functions in hepatitis B virus budding. Proc Natl Acad Sci USA. 2007;104(24):10205–10.
Google Scholar
WHO, Hepatitis D. 2023; https://www.who.int/news-room/fact-sheets/detail/hepatitis-d
Yurdaydın C, Idilman R, Bozkaya H, Bozdayi AM. Natural history and treatment of chronic delta hepatitis. J Viral Hepatitis. 2010;17(11):749–56.
Lombardo D, Franzè MS, Caminiti G, Pollicino T. Hepatitis delta virus and hepatocellular carcinoma. Pathogens (Basel Switzerland) 2024;13(5).
Smedile A, Farci P, Verme G, et al. Influence of delta infection on severity of hepatitis B. Lancet (London England). 1982;2(8305):945–7.
Google Scholar
Asselah T, Rizzetto M. Hepatitis D virus infection. N Engl J Med. 2023;389(1):58–70.
Google Scholar
Wu JC, Chen TZ, Huang YS, et al. Natural history of hepatitis D viral superinfection: significance of viremia detected by polymerase chain reaction. Gastroenterology. 1995;108(3):796–802.
Google Scholar
Schaper M, Rodriguez-Frias F, Jardi R, et al. Quantitative longitudinal evaluations of hepatitis delta virus RNA and hepatitis B virus DNA shows a dynamic, complex replicative profile in chronic hepatitis B and D. J Hepatol. 2010;52(5):658–64.
Google Scholar
Lucifora J, Alfaiate D, Pons C, et al. Hepatitis D virus interferes with hepatitis B virus RNA production via interferon-dependent and -independent mechanisms. J Hepatol. 2023;78(5):958–70.
Google Scholar
EASL Clinical Practice. Guidelines on hepatitis delta virus. J Hepatol. 2023;79(2):433–60.
Bai X, Chen L, Liu X et al. Adult hepatitis B virus vaccination coverage in China from 2011 to 2021: A systematic review. Vaccines 2022;10(6).
Pondé RAA, Amorim GSP. Elimination of the hepatitis B virus: A goal, a challenge. Med Res Rev 2024.
McAleer WJ, Buynak EB, Maigetter RZ, Wampler DE, Miller WJ, Hilleman MR. Human hepatitis B vaccine from Recombinant yeast. Nature. 1984;307(5947):178–80.
Google Scholar
Pattyn J, Hendrickx G, Vorsters A, Van Damme P, Hepatitis B, Vaccines. J Infect Dis. 2021;224(12 Suppl 2):S343–51.
Google Scholar
Pondé RAA. Expression and detection of anti-HBs antibodies after hepatitis B virus infection or vaccination in the context of protective immunity. Arch Virol. 2019;164(11):2645–58.
Google Scholar
Terrault NA, Lok ASF, McMahon BJ, et al. Update on prevention, diagnosis, and treatment of chronic hepatitis B: AASLD 2018 hepatitis B guidance. Clin Liver Disease. 2018;12(1):33–4.
EASL. 2017 Clinical Practice Guidelines on the management of hepatitis B virus infection. Journal of hepatology. 2017;67(2):370–398.
Blaney H, Khalid M, Heller T, Koh C. Epidemiology, presentation, and therapeutic approaches for hepatitis D infections. Expert Rev Anti Infect Ther. 2023;21(2):127–42.
Google Scholar
Elbahrawy A, Atalla H, Alboraie M et al. Recent advances in protective vaccines against hepatitis viruses: A narrative review. Viruses 2023;15(1).
Rizzetto M, Hamid S, Negro F. The changing context of hepatitis D. J Hepatol. 2021;74(5):1200–11.
Google Scholar
Matthews PC, Ocama P, Wang S, et al. Enhancing interventions for prevention of mother-to-child- transmission of hepatitis B virus. JHEP Reports: Innov Hepatol. 2023;5(8):100777.
Terrault NA, Lok ASF, McMahon BJ, et al. Update on prevention, diagnosis, and treatment of chronic hepatitis B: AASLD 2018 hepatitis B guidance. Hepatology (Baltimore MD). 2018;67(4):1560–99.
Google Scholar
WHO Guidelines Approved by the Guidelines Review Committee. In: Prevention of Mother-to-Child Transmission of Hepatitis B Virus: Guidelines on Antiviral Prophylaxis in Pregnancy. Geneva: World Health Organization © World Health Organization 2020.; 2020.
Kumar M, Abbas Z, Azami M, et al. Asian Pacific association for the study of liver (APASL) guidelines: hepatitis B virus in pregnancy. Hep Intl. 2022;16(2):211–53.
Dionne-Odom J, Tita AT, Silverman NS, #38. Hepatitis B in pregnancy screening, treatment, and prevention of vertical transmission. Am J Obstet Gynecol. 2016;214(1):6–14.
Gupta I, Ratho RK. Immunogenicity and safety of two schedules of hepatitis B vaccination during pregnancy. J Obstet Gynaecol Res. 2003;29(2):84–6.
Google Scholar
Xiao XM, Li AZ, Chen X, Zhu YK, Miao J. Prevention of vertical hepatitis B transmission by hepatitis B Immunoglobulin in the third trimester of pregnancy. Int J Gynaecol Obstet. 2007;96(3):167–70.
Google Scholar
Xu Q, Xiao L, Lu XB, Zhang YX, Cai X. A randomized controlled clinical trial: interruption of intrauterine transmission of hepatitis B virus infection with HBIG. World J Gastroenterol. 2006;12(21):3434–7.
Google Scholar
Eke AC, Eleje GU, Eke UA, Xia Y, Liu J. Hepatitis B Immunoglobulin during pregnancy for prevention of mother-to-child transmission of hepatitis B virus. Cochrane Database Syst Rev. 2017;2(2):Cd008545.
Google Scholar
Lee C, Gong Y, Brok J, Boxall EH, Gluud C. Effect of hepatitis B immunisation in newborn infants of mothers positive for hepatitis B surface antigen: systematic review and meta-analysis. BMJ (Clinical Res ed). 2006;332(7537):328–36.
Wong VC, Ip HM, Reesink HW, et al. Prevention of the HBsAg carrier state in newborn infants of mothers who are chronic carriers of HBsAg and hbeag by administration of hepatitis-B vaccine and hepatitis-B immunoglobulin. Double-blind randomised placebo-controlled study. Lancet (London England). 1984;1(8383):921–6.
Google Scholar
Pan CQ, Duan Z, Dai E, et al. Tenofovir to prevent hepatitis B transmission in mothers with high viral load. N Engl J Med. 2016;374(24):2324–34.
Google Scholar
Jourdain G, Ngo-Giang-Huong N, Harrison L, et al. Tenofovir versus placebo to prevent perinatal transmission of hepatitis B. N Engl J Med. 2018;378(10):911–23.
Google Scholar
Funk AL, Lu Y, Yoshida K, et al. Efficacy and safety of antiviral prophylaxis during pregnancy to prevent mother-to-child transmission of hepatitis B virus: a systematic review and meta-analysis. Lancet Infect Dis. 2021;21(1):70–84.
Google Scholar
Wen WH, Chen HL, Shih TT, et al. Long-term growth and bone development in children of HBV-infected mothers with and without fetal exposure to Tenofovir disoproxil fumarate. J Hepatol. 2020;72(6):1082–7.
Google Scholar
Zeng QL, Yu ZJ, Ji F, et al. Tenofovir Alafenamide to prevent perinatal hepatitis B transmission: A multicenter, prospective, observational study. Clin Infect Dis. 2021;73(9):e3324–32.
Google Scholar
Ding Y, Cao L, Zhu L, et al. Efficacy and safety of Tenofovir Alafenamide fumarate for preventing mother-to-child transmission of hepatitis B virus: a National cohort study. Aliment Pharmacol Ther. 2020;52(8):1377–86.
Google Scholar
Zeng QL, Zhang HX, Zhang JY, et al. Tenofovir Alafenamide for pregnant Chinese women with active chronic hepatitis B: A multicenter prospective study. Clin Gastroenterol Hepatol. 2022;20(12):2826–e28372829.
Google Scholar
Pan CQ, Zhu L, Yu AS, Zhao Y, Zhu B, Dai E. Tenofovir Alafenamide versus Tenofovir disoproxil fumarate for preventing vertical transmission in chronic hepatitis B mothers: A systematic review and Meta-Analysis. Clin Infect Dis. 2024;79(4):953–64.
Google Scholar
Sharma K, Murthy MK. A review of historical landmarks and pioneering technologies for the diagnosis of hepatitis C virus (HCV). Eur J Clin Microbiol Infect Dis. 2025;44(6):1289–303.
Google Scholar
Stevens A, Lafferty L, Treloar C, et al. Acceptability of hepatitis C testing using point-of-care testing and dried blood spot collection among people at risk of hepatitis C infection. Int J Drug Policy. 2025;137:104720.
Google Scholar
Conway A, Marshall AD, Grebely J, Fontaine G, Treloar C. Professional identities and new technologies of hepatitis C point-of-care testing. Soc Sci Med. 2025;378:118140.
Google Scholar
Debette-Gratien M, François S, Chevalier C, et al. Towards hepatitis C elimination in france: scanvir, an effective model to test and treat drug users on dedicated days. J Viral Hepat. 2023;30(4):355–61.
Google Scholar
Haga Y, Coates S, Ray R. Hepatitis C virus chronicity and oncogenic potential: vaccine development progress. Mol Aspects Med. 2024;99:101305.
Google Scholar
Liang TJ, Feld JJ, Cox AL, Rice CM. Controlled human infection Model – Fast track to HCV vaccine?? N Engl J Med. 2021;385(13):1235–40.
Google Scholar
Bartenschlager R, Baumert TF, Bukh J, et al. Critical challenges and emerging opportunities in hepatitis C virus research in an era of potent antiviral therapy: considerations for scientists and funding agencies. Virus Res. 2018;248:53–62.
Google Scholar
Chappell CA, Scarsi KK, Kirby BJ, et al. Ledipasvir plus Sofosbuvir in pregnant women with hepatitis C virus infection: a phase 1 Pharmacokinetic study. Lancet Microbe. 2020;1(5):E200–8.
Google Scholar
El-Sayed MH, Hassany M, Ebeid FSES, Zeidan A, Asem N. THU-136-Ledipasvir/sofosbuvir for 8 weeks cures genotype 4 chronic hepatitis C in non-cirrhotic children and adolescents. Journal of hepatology. 2019;70(1, Supplement):e221.
Kushner T, Terrault NA. Hepatitis C in pregnancy: A unique opportunity to improve the hepatitis C cascade of care. Hepatol Commun. 2019;3(1):20–8.
Google Scholar
Kushner T, Cohen J, Tien PC, Terrault NA. Evaluating women’s preferences for hepatitis C treatment during pregnancy. Hepatol Commun. 2018;2(11):1306–10.
Google Scholar
EASL Clinical Practice. Guidelines on the management of hepatitis B virus infection. J Hepatol 2025.
Lee HW, Lee JS, Ahn SH. Hepatitis B virus cure: targets and future therapies. Int J Mol Sci 2020;22(1).
Kim SK, Fujii T, Kim SR, et al. Hepatitis B virus treatment and hepatocellular carcinoma: controversies and approaches to consensus. Liver Cancer. 2022;11(6):497–510.
Google Scholar
Papatheodoridis GV, Idilman R, Dalekos GN, et al. The risk of hepatocellular carcinoma decreases after the first 5 years of Entecavir or Tenofovir in Caucasians with chronic hepatitis B. Hepatology (Baltimore MD). 2017;66(5):1444–53.
Google Scholar
Yuen MF, Chen DS, Dusheiko GM, et al. Hepatitis B virus infection. Nat Reviews Disease Primers. 2018;4:18035.
Google Scholar
Lok AS, McMahon BJ, Brown RS Jr., et al. Antiviral therapy for chronic hepatitis B viral infection in adults: A systematic review and meta-analysis. Hepatology (Baltimore MD). 2016;63(1):284–306.
Google Scholar
Petersen J, Thompson AJ, Levrero M. Aiming for cure in HBV and HDV infection. J Hepatol. 2016;65(4):835–48.
Google Scholar
Pan Y, Xia H, He Y, Zeng S, Shen Z, Huang W. The progress of molecules and strategies for the treatment of HBV infection. Front Cell Infect Microbiol. 2023;13:1128807.
Google Scholar
Buti M, Marcos-Fosch C, Esteban R. Nucleos(t)ide analogue therapy: the role of Tenofovir Alafenamide. Liver International: Official J Int Association Study Liver. 2021;41(Suppl 1):9–14.
Menéndez-Arias L, Álvarez M, Pacheco B. Nucleoside/nucleotide analog inhibitors of hepatitis B virus polymerase: mechanism of action and resistance. Curr Opin Virol. 2014;8:1–9.
Google Scholar
Levrero M, Subic M, Villeret F, Zoulim F. Perspectives and limitations for nucleo(t)side analogs in future HBV therapies. Curr Opin Virol. 2018;30:80–9.
Google Scholar
Chang TT, Gish RG, de Man R, et al. A comparison of Entecavir and lamivudine for HBeAg-positive chronic hepatitis B. N Engl J Med. 2006;354(10):1001–10.
Google Scholar
Marcellin P, Heathcote EJ, Buti M, et al. Tenofovir disoproxil fumarate versus Adefovir dipivoxil for chronic hepatitis B. N Engl J Med. 2008;359(23):2442–55.
Google Scholar
Chang TT, Lai CL, Kew Yoon S, et al. Entecavir treatment for up to 5 years in patients with hepatitis B e antigen-positive chronic hepatitis B. Hepatology (Baltimore MD). 2010;51(2):422–30.
Google Scholar
Buti M, Tsai N, Petersen J, et al. Seven-year efficacy and safety of treatment with Tenofovir disoproxil fumarate for chronic hepatitis B virus infection. Dig Dis Sci. 2015;60(5):1457–64.
Google Scholar
Yardeni D, Chang KM, Ghany MG. Current best practice in hepatitis B management and Understanding Long-term prospects for cure. Gastroenterology. 2023;164(1):42–e6046.
Google Scholar
World Health O. Guidelines for the prevention, care and treatment of persons with chronic hepatitis B infection. Geneva: World Health Organization; 2015.
Wong GL, Tse YK, Wong VW, Yip TC, Tsoi KK, Chan HL. Long-term safety of oral nucleos(t)ide analogs for patients with chronic hepatitis B: A cohort study of 53,500 subjects. Hepatology (Baltimore MD). 2015;62(3):684–93.
Google Scholar
Hsu YC, Wei MT, Nguyen MH. Tenofovir Alafenamide as compared to Tenofovir disoproxil fumarate in the management of chronic hepatitis B with recent trends in patient demographics. Expert Rev Gastroenterol Hepatol. 2017;11(11):999–1008.
Google Scholar
Yuen MF, Lai CL. Treatment of chronic hepatitis B: evolution over two decades. J Gastroenterol Hepatol. 2011;26(Suppl 1):138–43.
Google Scholar
Petersen J, Heyne R, Mauss S, et al. Effectiveness and safety of Tenofovir disoproxil fumarate in chronic hepatitis B: A 3-Year prospective field practice study in Germany. Dig Dis Sci. 2016;61(10):3061–71.
Google Scholar
Nijampatnam B, Liotta DC. Recent advances in the development of HBV capsid assembly modulators. Curr Opin Chem Biol. 2019;50:73–9.
Google Scholar
McFadden WM, Sarafianos SG. Biology of the hepatitis B virus (HBV) core and capsid assembly modulators (CAMs) for chronic hepatitis B (CHB) cure. Global Health Med. 2023;5(4):199–207.
Amblard F, Chen Z, Wiseman J, et al. Synthesis and evaluation of highly potent HBV capsid assembly modulators (CAMs). Bioorg Chem. 2023;141:106923.
Google Scholar
Zhang M, Gao Y, Kong F, et al. Efficacy and safety of GLS4 with Entecavir vs Entecavir alone in chronic hepatitis B patients: A multicenter clinical trial. J Infect. 2025;90(3):106446.
Google Scholar
Jia H, Mai J, Wu M, et al. Safety, tolerability, pharmacokinetics, and antiviral activity of the novel core protein allosteric modulator ZM-H1505R (Canocapavir) in chronic hepatitis B patients: a randomized multiple-dose escalation trial. BMC Med. 2023;21(1):98.
Google Scholar
Zheng Y, Yang L, Yu L et al. Canocapavir is a novel capsid assembly modulator inducing a conformational change of the linker region of HBV core protein. Viruses 2023;15(5).
Vendeville S, Amblard F, Bassit L, et al. The discovery and preclinical profile of ALG-000184, a prodrug of the potent hepatitis B virus capsid assembly modulator ALG-001075. J Med Chem. 2024;67(23):21126–42.
Google Scholar
Allweiss L, Volmari A, Suri V, et al. Blocking viral entry with bulevirtide reduces the number of HDV-infected hepatocytes in human liver biopsies. J Hepatol. 2024;80(6):882–91.
Google Scholar
Liu H, Zakrzewicz D, Nosol K, et al. Structure of antiviral drug bulevirtide bound to hepatitis B and D virus receptor protein NTCP. Nat Commun. 2024;15(1):2476.
Google Scholar
Urban S, Bartenschlager R, Kubitz R, Zoulim F. Strategies to inhibit entry of HBV and HDV into hepatocytes. Gastroenterology. 2014;147(1):48–64.
Google Scholar
Wedemeyer H, Bogomolov P, Blank A, et al. Final results of a multicenter, open-label phase 2b clinical trial to assess safety and efficacy of myrcludex B in combination with Tenofovir in patients with chronic HBV/HDV co-infection. J Hepatol. 2018;68:S3–3.
Loglio A, Ferenci P, Uceda Renteria SC, et al. Excellent safety and effectiveness of high-dose myrcludex-B monotherapy administered for 48 weeks in HDV-related compensated cirrhosis: A case report of 3 patients. J Hepatol. 2019;71(4):834–9.
Google Scholar
Kim SW, Yoon JS, Lee M, Cho Y. Toward a complete cure for chronic hepatitis B: novel therapeutic targets for hepatitis B virus. Clin Mol Hepatol. 2022;28(1):17–30.
Google Scholar
Hosaka T, Suzuki F, Kobayashi M, et al. Long-term Entecavir treatment reduces hepatocellular carcinoma incidence in patients with hepatitis B virus infection. Hepatology (Baltimore MD). 2013;58(1):98–107.
Google Scholar
Coffin CS, Rezaeeaval M, Pang JX, et al. The incidence of hepatocellular carcinoma is reduced in patients with chronic hepatitis B on long-term nucleos(t)ide analogue therapy. Aliment Pharmacol Ther. 2014;40(11–12):1262–9.
Google Scholar
Li H, Sheng C, Wang S, et al. Removal of integrated hepatitis B virus DNA using CRISPR-Cas9. Front Cell Infect Microbiol. 2017;7:91.
Google Scholar
Kostyushev D, Kostyusheva A, Brezgin S, et al. Suppressing the NHEJ pathway by DNA-PKcs inhibitor NU7026 prevents degradation of HBV CccDNA cleaved by CRISPR/Cas9. Sci Rep. 2019;9(1):1847.
Google Scholar
Bloom K, Ely A, Mussolino C, Cathomen T, Arbuthnot P. Inactivation of hepatitis B virus replication in cultured cells and in vivo with engineered transcription activator-like effector nucleases. Mol Therapy: J Am Soc Gene Therapy. 2013;21(10):1889–97.
Chen J, Zhang W, Lin J, et al. An efficient antiviral strategy for targeting hepatitis B virus genome using transcription activator-like effector nucleases. Mol Therapy: J Am Soc Gene Therapy. 2014;22(2):303–11.
Hong J, Rajwanshi VK. Antisense oligonucleotides as drugs with both direct and indirect antiviral actions. Antiviral Res. 2025;240:106219.
Google Scholar
Yuen MF, Lim SG, Plesniak R, et al. Efficacy and safety of bepirovirsen in chronic hepatitis B infection. N Engl J Med. 2022;387(21):1957–68.
Google Scholar
Gao YH, Liang XE, Tan YW et al. HBsAg loss and seroconversion after 16-week or 24-week AHB-137 treatment in HBeAg-negative chronic hepatitis B participants on NA therapy: results from an ongoing multicenter, randomized phase IIb study. J Hepatol 2025;82.
Grünweller A, Hartmann RK. RNA interference as a gene-specific approach for molecular medicine. Curr Med Chem. 2005;12(26):3143–61.
Google Scholar
Ryther RC, Flynt AS, Phillips JA 3rd, Patton JG. SiRNA therapeutics: big potential from small RNAs. Gene Ther. 2005;12(1):5–11.
Google Scholar
Hui RW, Mak LY, Seto WK, Yuen MF. RNA interference as a novel treatment strategy for chronic hepatitis B infection. Clin Mol Hepatol. 2022;28(3):408–24.
Google Scholar
Sneller L, Lin C, Price A, Kottilil S, Chua JV. RNA interference therapeutics for chronic hepatitis B: progress, challenges, and future prospects. Microorganisms 2024;12(3).
MacRae IJ, Ma E, Zhou M, Robinson CV, Doudna JA. In vitro reconstitution of the human RISC-loading complex. Proc Natl Acad Sci U S A. 2008;105(2):512–7.
Google Scholar
Macrae IJ, Zhou K, Li F, et al. Structural basis for double-stranded RNA processing by Dicer. Science. 2006;311(5758):195–8.
Google Scholar
Sajid MI, Moazzam M, Kato S, Yeseom Cho K, Tiwari RK. Overcoming barriers for SiRNA therapeutics: from bench to bedside. Pharmaceuticals (Basel) 2020;13(10).
Qiu Y, Lam JK, Leung SW, Liang W. Delivery of RNAi therapeutics to the Airways-From bench to bedside. Molecules 2016;21(9).
Aagaard L, Rossi JJ. RNAi therapeutics: principles, prospects and challenges. Adv Drug Deliv Rev. 2007;59(2–3):75–86.
Google Scholar
Iwakawa HO, Tomari Y. Life of RISC: formation, action, and degradation of RNA-induced Silencing complex. Mol Cell. 2022;82(1):30–43.
Google Scholar
Thi EP, Dhillon AP, Ardzinski A, et al. ARB-1740, a RNA interference therapeutic for chronic hepatitis B infection. ACS Infect Dis. 2019;5(5):725–37.
Google Scholar
Sajid MI, Moazzam M, Cho Y, et al. SiRNA therapeutics for the therapy of COVID-19 and other coronaviruses. Mol Pharm. 2021;18(6):2105–21.
Google Scholar
Kang H, Ga YJ, Kim SH, et al. Small interfering RNA (siRNA)-based therapeutic applications against viruses: principles, potential, and challenges. J Biomed Sci. 2023;30(1):88.
Google Scholar
Nguyen L, Nguyen TT, Kim JY, Jeong JH. Advanced SiRNA delivery in combating hepatitis B virus: mechanistic insights and recent updates. J Nanobiotechnol. 2024;22(1):745.
Wooddell CI, Yuen MF, Chan HL et al. RNAi-based treatment of chronically infected patients and chimpanzees reveals that integrated hepatitis B virus DNA is a source of HBsAg. Sci Transl Med 2017;9(409).
Lee ACH, Thi EP, Cuconati A, et al. Function and drug combination studies in cell culture models for AB-729, a subcutaneously administered SiRNA investigational agent for chronic hepatitis B infection. J Hepatol. 2019;70(1):E471–471.
Streinu-Cercel A, Gane E, Cheng W, et al. SAT-155 – A phase 2a study evaluating the multi-dose activity of ARB-1467 in hbeag positive and negative virally suppressed subjects with hepatitis B. J Hepatol. 2017;66(1):S688–9.
Hou J, Zhang W, Xie Q et al. Xalnesiran with or without an Immunomodulator in chronic hepatitis B. 2024;391(22):2098–109.
Yuen MF, Lim YS, Yoon KT, et al. VIR-2218 (elebsiran) plus pegylated interferon-alfa-2a in participants with chronic hepatitis B virus infection: a phase 2 study. Lancet Gastroenterol Hepatol. 2024;9(12):1121–32.
Google Scholar
Agarwal K, Buti M, van Bömmel F, et al. JNJ-73763989 and Bersacapavir treatment in nucleos(t)ide analogue-suppressed patients with chronic hepatitis B: REEF-2. J Hepatol. 2024;81(3):404–14.
Google Scholar
Gane EJ, Kim W, Lim TH, et al. First-in-human randomized study of RNAi therapeutic RG6346 for chronic hepatitis B virus infection. J Hepatol. 2023;79(5):1139–49.
Google Scholar
Iannacone M, Beccaria CG, Allweiss L, et al. Targeting HBV with RNA interference: paths to cure. Sci Transl Med. 2025;17(805):eadv3678.
Google Scholar
Vaillant A. REP 2139: antiviral mechanisms and applications in achieving functional control of HBV and HDV infection. ACS Infect Dis. 2019;5(5):675–87.
Google Scholar
Blanchet M, Sinnathamby V, Vaillant A, Labonté P. Inhibition of HBsAg secretion by nucleic acid polymers in HepG2.2.15 cells. Antiviral Res. 2019;164:97–105.
Google Scholar
Hershkovich L, Shekhtman L, Bazinet M et al. Rapid monophasic HBsAg decline during nucleic-acid polymer-based therapy predicts functional cure. Hepatol Commun 2023;7(8).
Boulon R, Blanchet M, Lemasson M, Vaillant A, Labonté P. Characterization of the antiviral effects of REP 2139 on the HBV lifecycle in vitro. Antiviral Res. 2020;183:104853.
Google Scholar
Brillanti S. Management of delta hepatitis 45 years after the discovery of HDV. J Clin Med 2022;11(6).
Bazinet M, Pântea V, Cebotarescu V, et al. Safety and efficacy of REP 2139 and pegylated interferon alfa-2a for treatment-naive patients with chronic hepatitis B virus and hepatitis D virus co-infection (REP 301 and REP 301-LTF): a non-randomised, open-label, phase 2 trial. Lancet Gastroenterol Hepatol. 2017;2(12):877–89.
Google Scholar
Bazinet M, Pântea V, Placinta G, et al. Safety and efficacy of 48 weeks REP 2139 or REP 2165, Tenofovir disoproxil, and pegylated interferon Alfa-2a in patients with chronic HBV infection Naïve to Nucleos(t)ide therapy. Gastroenterology. 2020;158(8):2180–94.
Google Scholar
Watanabe T, Hayashi S, Zhaoyu Y, et al. A novel, small anti-HBV compound reduces HBsAg and HBV-DNA by destabilizing HBV-RNA. J Gastroenterol. 2024;59(4):315–28.
Google Scholar
Lam AM, Dugyala RR, Sheraz M et al. Preclinical antiviral and safety profiling of the HBV RNA destabilizer AB-161. Viruses 2024;16(3).
Lucifora J, Xia Y, Reisinger F, et al. Specific and nonhepatotoxic degradation of nuclear hepatitis B virus CccDNA. Sci (New York NY). 2014;343(6176):1221–8.
Li Q, Sun B, Zhuo Y, et al. Interferon and interferon-stimulated genes in HBV treatment. Front Immunol. 2022;13:1034968.
Google Scholar
Li F, Qu L, Liu Y, et al. PegIFN alpha-2a reduces relapse in HBeAg-negative patients after nucleo(s)tide analogue cessation: A randomized-controlled trial. J Hepatol. 2025;82(2):211–21.
Google Scholar
Lang T, Lo C, Skinner N, Locarnini S, Visvanathan K, Mansell A. The hepatitis B e antigen (HBeAg) targets and suppresses activation of the toll-like receptor signaling pathway. J Hepatol. 2011;55(4):762–9.
Google Scholar
Jo J, Tan AT, Ussher JE, et al. Toll-like receptor 8 agonist and bacteria trigger potent activation of innate immune cells in human liver. PLoS Pathog. 2014;10(6):e1004210.
Google Scholar
Alexopoulou A, Vasilieva L, Karayiannis P. New approaches to the treatment of chronic hepatitis B. J Clin Med 2020;9(10).
Niu C, Li L, Daffis S, et al. Toll-like receptor 7 agonist GS-9620 induces prolonged Inhibition of HBV via a type I interferon-dependent mechanism. J Hepatol. 2018;68(5):922–31.
Google Scholar
Menne S, Tumas DB, Liu KH, et al. Sustained efficacy and seroconversion with the Toll-like receptor 7 agonist GS-9620 in the woodchuck model of chronic hepatitis B. J Hepatol. 2015;62(6):1237–45.
Google Scholar
Lanford RE, Guerra B, Chavez D, et al. GS-9620, an oral agonist of Toll-like receptor-7, induces prolonged suppression of hepatitis B virus in chronically infected chimpanzees. Gastroenterology. 2013;144(7):1508–17. 1517.e.
Google Scholar
Daffis S, Balsitis S, Chamberlain J, et al. Toll-Like receptor 8 agonist GS-9688 induces sustained efficacy in the woodchuck model of chronic hepatitis B. Hepatology (Baltimore MD). 2021;73(1):53–67.
Google Scholar
Dawood A, Abdul Basit S, Jayaraj M, Gish RG. Drugs in development for hepatitis B. Drugs. 2017;77(12):1263–80.
Google Scholar
Yuen MF, Chen CY, Liu CJ, et al. A phase 2, open-label, randomized, multiple-dose study evaluating inarigivir in treatment-naïve patients with chronic hepatitis B. Liver International: Official J Int Association Study Liver. 2023;43(1):77–89.
Yuen M-F, Chen C-Y, Liu C-J, et al. GS-12-Ascending dose cohort study of inarigivir – A novel RIG I agonist in chronic HBV patients: final results of the ACHIEVE trial. J Hepatol. 2019;70(1):e47–8.
Agarwal K, Afdhal N, Coffin C, et al. Liver toxicity in the phase 2 catalyst 206 trial of inarigivir 400 mg daily added to a nucleoside in HBV EAg negative patients. J Hepatol. 2020;73:S125–125.
Peng G, Li S, Wu W, Tan X, Chen Y, Chen Z. PD-1 upregulation is associated with HBV-specific T cell dysfunction in chronic hepatitis B patients. Mol Immunol. 2008;45(4):963–70.
Google Scholar
Kassel R, Cruise MW, Iezzoni JC, Taylor NA, Pruett TL, Hahn YS. Chronically inflamed livers up-regulate expression of inhibitory B7 family members. Hepatology (Baltimore MD). 2009;50(5):1625–37.
Google Scholar
Zhang WJ, Peng CH, Zheng SS. Programmed death 1 and programmed death ligand 1 expressions in patients with chronic hepatitis B. Hepatobiliary Pancreat Dis International: HBPD INT. 2013;12(4):394–9.
Gane E, Verdon DJ, Brooks AE, et al. Anti-PD-1 Blockade with nivolumab with and without therapeutic vaccination for virally suppressed chronic hepatitis B: A pilot study. J Hepatol. 2019;71(5):900–7.
Google Scholar
Lee YB, Lee JH, Kim YJ, Yoon JH, Lee HS. The effect of therapeutic vaccination for the treatment of chronic hepatitis B virus infection. J Med Virol. 2015;87(4):575–82.
Google Scholar
Zoulim F, Fournier C, Habersetzer F, et al. Safety and immunogenicity of the therapeutic vaccine TG1050 in chronic hepatitis B patients: a phase 1b placebo-controlled trial. Hum Vaccines Immunotherapeutics. 2020;16(2):388–99.
Lok AS, Pan CQ, Han SH, et al. Randomized phase II study of GS-4774 as a therapeutic vaccine in virally suppressed patients with chronic hepatitis B. J Hepatol. 2016;65(3):509–16.
Google Scholar
Li C, Lee A, Grigoryan L, et al. Mechanisms of innate and adaptive immunity to the Pfizer-BioNTech BNT162b2 vaccine. Nat Immunol. 2022;23(4):543–55.
Google Scholar
Pardi N, Krammer F. mRNA vaccines for infectious diseases – advances, challenges and opportunities. Nat Rev Drug Discovery. 2024;23(11):838–61.
Google Scholar
Tseng HF, Ackerson BK, Sy LS, et al. mRNA-1273 bivalent (original and Omicron) COVID-19 vaccine effectiveness against COVID-19 outcomes in the united States. Nat Commun. 2023;14(1):5851.
Google Scholar
Narayanan E, Falcone S, Elbashir SM et al. Rational design and in vivo characterization of mRNA-Encoded broadly neutralizing antibody combinations against HIV-1. Antibodies (Basel Switzerland) 2022;11(4).
Lee IT, Nachbagauer R, Ensz D, et al. Safety and immunogenicity of a phase 1/2 randomized clinical trial of a quadrivalent, mRNA-based seasonal influenza vaccine (mRNA-1010) in healthy adults: interim analysis. Nat Commun. 2023;14(1):3631.
Google Scholar
Zhao H, Shao X, Yu Y, et al. A therapeutic hepatitis B mRNA vaccine with strong immunogenicity and persistent virological suppression. NPJ Vaccines. 2024;9(1):22.
Google Scholar
Hoofnagle JH, Mullen KD, Jones DB, et al. Treatment of chronic non-A,non-B hepatitis with Recombinant human alpha interferon. A preliminary report. N Engl J Med. 1986;315(25):1575–8.
Google Scholar
Fried MW, Shiffman ML, Reddy KR, et al. Peginterferon alfa-2a plus ribavirin for chronic hepatitis C virus infection. N Engl J Med. 2002;347(13):975–82.
Google Scholar
Di Marco L, Cannova S, Ferrigno E et al. A comprehensive review of antiviral therapy for hepatitis C: the long journey from interferon to Pan-Genotypic Direct-Acting antivirals (DAAs). Viruses 2025;17(2).
Taha G, Ezra L, Abu-Freha N, Hepatitis C. Elimination: opportunities and challenges in 2023. Viruses 2023;15(7).
Tani J, Masaki T, Oura K, Tadokoro T, Morishita A, Kobara H. Extrahepatic cancer risk in patients with hepatitis C virus infection treated with Direct-Acting antivirals. Microorganisms 2024;12(9).
Garbuglia AR, Pauciullo S, Zulian V, Del Porto P. Update on hepatitis C vaccine: results and challenges. Viruses 2024;16(8).
Zhou J, Wang FD, Li LQ, Chen EQ. Management of in- and out-of-hospital screening for hepatitis C. Front Public Health. 2022;10:984810.
Google Scholar
Götte M, Feld JJ. Direct-acting antiviral agents for hepatitis C: structural and mechanistic insights. Nat Reviews Gastroenterol Hepatol. 2016;13(6):338–51.
Lamarre D, Anderson PC, Bailey M, et al. An NS3 protease inhibitor with antiviral effects in humans infected with hepatitis C virus. Nature. 2003;426(6963):186–9.
Google Scholar
Hinrichsen H, Benhamou Y, Wedemeyer H, et al. Short-term antiviral efficacy of BILN 2061, a hepatitis C virus Serine protease inhibitor, in hepatitis C genotype 1 patients. Gastroenterology. 2004;127(5):1347–55.
Google Scholar
Vanwolleghem T, Meuleman P, Libbrecht L, Roskams T, De Vos R, Leroux-Roels G. Ultra-rapid cardiotoxicity of the hepatitis C virus protease inhibitor BILN 2061 in the urokinase-type plasminogen activator mouse. Gastroenterology. 2007;133(4):1144–55.
Google Scholar
Poordad F, McCone J Jr., Bacon BR, et al. Boceprevir for untreated chronic HCV genotype 1 infection. N Engl J Med. 2011;364(13):1195–206.
Google Scholar
Jacobson IM, McHutchison JG, Dusheiko G, et al. Telaprevir for previously untreated chronic hepatitis C virus infection. N Engl J Med. 2011;364(25):2405–16.
Google Scholar
Romano KP, Ali A, Royer WE, Schiffer CA. Drug resistance against HCV NS3/4A inhibitors is defined by the balance of substrate recognition versus inhibitor binding. Proc Natl Acad Sci USA. 2010;107(49):20986–91.
Google Scholar
Lawitz E, Jacobson IM, Nelson DR, et al. Development of Sofosbuvir for the treatment of hepatitis C virus infection. Ann N Y Acad Sci. 2015;1358:56–67.
Google Scholar
Abraham GM, Spooner LM. Sofosbuvir in the treatment of chronic hepatitis C: new dog, new tricks. Clin Infect Diseases: Official Publication Infect Dis Soc Am. 2014;59(3):411–5.
Sofosbuvir/Velpatasvir. (Epclusa) for hepatitis C. JAMA. 2017;317(6):639–40.
Younossi ZM, Stepanova M, Sulkowski M, Wyles D, Kottilil S, Hunt S. Patient-reported outcomes in patients co-infected with hepatitis C virus and human immunodeficiency virus treated with Sofosbuvir and velpatasvir: the ASTRAL-5 study. Liver International: Official J Int Association Study Liver. 2017;37(12):1796–804.
EASL Recommendations on Treatment of Hepatitis C. 2018. Journal of hepatology. 2018;69(2):461–511.
Hepatitis CG. 2018 Update: AASLD-IDSA Recommendations for Testing, Managing, and Treating Hepatitis C Virus Infection. Clin Infect Dis. 2018;67(10):1477–1492.
Bourlière M, Gordon SC, Flamm SL, et al. Sofosbuvir, velpatasvir, and voxilaprevir for previously treated HCV infection. N Engl J Med. 2017;376(22):2134–46.
Google Scholar
Degasperi E, Spinetti A, Lombardi A, et al. Real-life effectiveness and safety of sofosbuvir/velpatasvir/voxilaprevir in hepatitis C patients with previous DAA failure. J Hepatol. 2019;71(6):1106–15.
Google Scholar
Llaneras J, Riveiro-Barciela M, Lens S, et al. Effectiveness and safety of sofosbuvir/velpatasvir/voxilaprevir in patients with chronic hepatitis C previously treated with DAAs. J Hepatol. 2019;71(4):666–72.
Google Scholar
Graf C, D’Ambrosio R, Degasperi E, et al. Real-world effectiveness of voxilaprevir/velpatasvir/sofosbuvir in patients following DAA failure. JHEP Reports: Innov Hepatol. 2024;6(3):100994.
Lampertico P, Carrión JA, Curry M, et al. Real-world effectiveness and safety of glecaprevir/pibrentasvir for the treatment of patients with chronic HCV infection: A meta-analysis. J Hepatol. 2020;72(6):1112–21.
Google Scholar
Lamb YN. Glecaprevir/Pibrentasvir: first global approval. Drugs. 2017;77(16):1797–804.
Google Scholar
Wedemeyer H, Erren P, Naumann U, et al. Glecaprevir/pibrentasvir is safe and effective in hepatitis C patients with cirrhosis: Real-world data from the German hepatitis C-Registry. Liver International: Official J Int Association Study Liver. 2021;41(5):949–55.
Lu M, Rupp LB, Melkonian C, et al. Real-World safety and effectiveness of an 8-Week regimen of glecaprevir/pibrentasvir in patients with hepatitis C and cirrhosis. Adv Therapy. 2024;41(2):744–58.
Pol S, Thompson AJ, Collins M et al. Effectiveness and safety of glecaprevir/pibrentasvir for 8 weeks in the treatment of patients with acute hepatitis C: A single-arm retrospective study. Hepatology (Baltimore MD). 2024.
Brown RS Jr., Buti M, Rodrigues L, et al. Glecaprevir/pibrentasvir for 8 weeks in treatment-naïve patients with chronic HCV genotypes 1–6 and compensated cirrhosis: the EXPEDITION-8 trial. J Hepatol. 2020;72(3):441–9.
Google Scholar
Wei L, Wang G, Alami NN, et al. Glecaprevir-pibrentasvir to treat chronic hepatitis C virus infection in asia: two multicentre, phase 3 studies- a randomised, double-blind study (VOYAGE-1) and an open-label, single-arm study (VOYAGE-2). Lancet Gastroenterol Hepatol. 2020;5(9):839–49.
Google Scholar
Zhou XJ, Good SS, Pietropaolo K, et al. Bemnifosbuvir (BEM, AT-527), a novel nucleotide analogue inhibitor of the hepatitis C virus NS5B polymerase. Expert Opin Investig Drugs. 2024;33(1):9–17.
Google Scholar
Good SS, Moussa A, Zhou XJ, Pietropaolo K, Sommadossi JP. Preclinical evaluation of AT-527, a novel Guanosine nucleotide prodrug with potent, pan-genotypic activity against hepatitis C virus. PLoS ONE. 2020;15(1):e0227104.
Google Scholar
Jucov A, Conway B, Iliescu L, et al. THU-382 Lead-in cohort results from a phase 2 study of a novel 8-week combination regimen of bemnifosbuvir and Ruzasvir in patients with chronic hepatitis C virus infection. J Hepatol. 2024;80:S819.
Castelnau C, Le Gal F, Ripault MP, et al. Efficacy of peginterferon alpha-2b in chronic hepatitis delta: relevance of quantitative RT-PCR for follow-up. Hepatology (Baltimore MD). 2006;44(3):728–35.
Google Scholar
Heidrich B, Yurdaydın C, Kabaçam G, et al. Late HDV RNA relapse after peginterferon alpha-based therapy of chronic hepatitis delta. Hepatology (Baltimore MD). 2014;60(1):87–97.
Google Scholar
Kotenko SV, Gallagher G, Baurin VV, et al. IFN-lambdas mediate antiviral protection through a distinct class II cytokine receptor complex. Nat Immunol. 2003;4(1):69–77.
Google Scholar
Sheppard P, Kindsvogel W, Xu W, et al. IL-28, IL-29 and their class II cytokine receptor IL-28R. Nat Immunol. 2003;4(1):63–8.
Google Scholar
Giersch K, Homs M, Volz T, et al. Both interferon alpha and lambda can reduce all intrahepatic HDV infection markers in HBV/HDV infected humanized mice. Sci Rep. 2017;7(1):3757.
Google Scholar
Kang C, Syed YY. Bulevirtide: first approval. Drugs. 2020;80(15):1601–5.
Google Scholar
Wedemeyer H, Schöneweis K, Bogomolov P, et al. Safety and efficacy of bulevirtide in combination with Tenofovir disoproxil fumarate in patients with hepatitis B virus and hepatitis D virus coinfection (MYR202): a multicentre, randomised, parallel-group, open-label, phase 2 trial. Lancet Infect Dis. 2023;23(1):117–29.
Google Scholar
Wedemeyer H, Aleman S, Brunetto MR, et al. A phase 3, randomized trial of bulevirtide in chronic hepatitis D. N Engl J Med. 2023;389(1):22–32.
Google Scholar
Dhillon S. Lonafarnib: first approval. Drugs. 2021;81(2):283–9.
Google Scholar
Lange F, Garn J, Anagho HA, et al. Hepatitis D virus infection, innate immune response and antiviral treatments in stem cell-derived hepatocytes. Liver International: Official J Int Association Study Liver. 2023;43(10):2116–29.
Liu M, Bryant MS, Chen J, et al. Antitumor activity of SCH 66336, an orally bioavailable tricyclic inhibitor of Farnesyl protein transferase, in human tumor xenograft models and wap-ras Transgenic mice. Cancer Res. 1998;58(21):4947–56.
Google Scholar
Roca Suarez AA, Batbold E, Bartosch B, Dashdorj N, Testoni B, Zoulim F. Emerging anti-HDV drugs and HBV cure strategies with anti-HDV activity. Liver International: Official J Int Association Study Liver. 2023;43(Suppl 1):87–95.
Hossain MA. Targeting the RAS upstream and downstream signaling pathway for cancer treatment. Eur J Pharmacol. 2024;979:176727.
Google Scholar
Einav S, Glenn JS. Prenylation inhibitors: a novel class of antiviral agents. J Antimicrob Chemother. 2003;52(6):883–6.
Google Scholar
Yurdaydin C, Keskin O, Kalkan Ç, et al. Optimizing Lonafarnib treatment for the management of chronic delta hepatitis: the LOWR HDV-1 study. Hepatology (Baltimore MD). 2018;67(4):1224–36.
Google Scholar
Yurdaydin C, Idilman R, Keskin O, et al. A phase 2 dose-optimization study of Lonafarnib with Ritonavir for the treatment of chronic delta hepatitis-end of treatment results from the LOWR HDV-2 study. J Hepatol. 2017;66(1):S33–4.
Koh C, Surana P, Han T, et al. A phase 2 study exploring once daily dosing of Ritonavir boosted Lonafarnib for the treatment of chronic delta hepatitis – end of study results from the LOWR HDV-3 study. J Hepatol. 2017;66(1):S101–2.
Wedemeyer H, Port K, Deterding K, et al. A phase 2 dose-escalation study of Lonafarnib plus Ritonavir in patients with chronic hepatitis D: final results from the Lonafarnib with Ritonavir in HDV-4 (LOWR HDV-4) study. J Hepatol. 2017;66(1):S24–24.