Synaptic proteins degrade more slowly in aged mice than in younger mice, a new study finds. Microglia appear to unburden the neurons of the excess proteins, but that accumulation may turn toxic, the findings suggest.
To function…
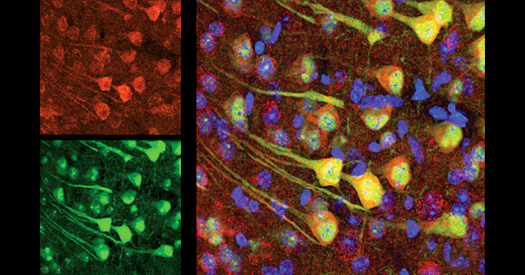
Synaptic proteins degrade more slowly in aged mice than in younger mice, a new study finds. Microglia appear to unburden the neurons of the excess proteins, but that accumulation may turn toxic, the findings suggest.
To function…
For years, women died of…
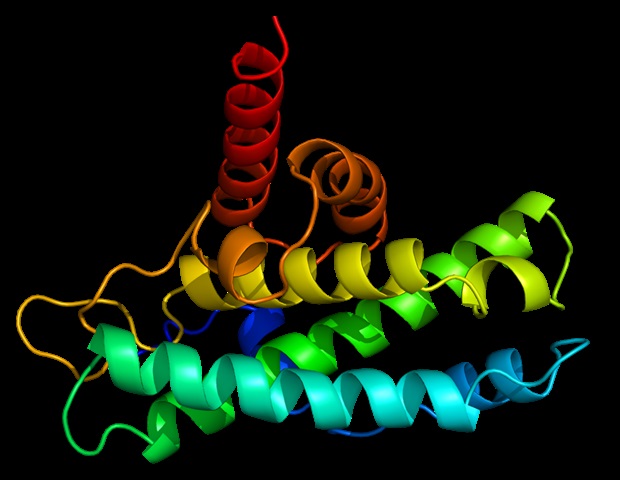
Cornell researchers have discovered a new way cells regulate how they respond to stress, identifying an interaction between two proteins that helps keep a critical cellular recycling system in balance.
The findings show that a…


Researchers at Nankai University in Tianjin have identified a breakthrough biological target for treating age-related infertility.
The study, published in Cell Reports Medicine, offers a fresh perspective on why…
Feb 9 : Artificial intelligence tools are more likely to provide incorrect medical advice when the misinformation comes from what the software considers to be an authoritative source, a new study found.
In tests of 20 open-source and proprietary…
Invasive aspergillosis (IA) remains a serious and often fatal infection despite advances in diagnostics and antifungal therapy. Although mold-active triazoles have long been recommended as first-line therapy, liposomal amphotericin B continues…

In patients with severe mitral regurgitation (MR), low left atrial (LA) reservoir strain (LASr) and prior atrial fibrillation (AFib) intervention or LA appendage (LAA) ligation may predict low invasive LA compliance and limited V-wave reduction…

“Hidden Risk in a Common Fruit: Bananas Contaminated by Mining Disaster”, 09 February 2026
…A team of researchers in soil geochemistry, environmental engineering, and health from the University of São Paulo (USP), the Federal University of…