The French Territories of the Americas are regions frequently impacted by arthropod-borne viruses transmitted by Aedes aegypti mosquitoes, such as dengue, chikungunya, and Zika viruses [1]. However, the presence of Aedes albopictus, another important mosquito vector for arboviruses [2], had never been reported in any of these territories, despite the impressive spread of this mosquito in mainland France since 2004 [3]. In the Caribbean, Ae. albopictus was first reported in the Dominican Republic in 1993 [4].
On 30 September 2024, two vector control agents collected mosquito larvae from a floor siphon (17°54′20″N, 62°49′28″W) near the Lorient post office on the island of Saint Barthélemy, as part of their routine entomological surveillance activities that include weekly larval prospections and ovitrapping. The larvae were brought to the Vector Control Agency office (Agence Régionale de Santé [ARS]) in Saint Barthélemy and reared until adulthood. After emergence, the agents noted the presence of adult mosquitoes that did not match morphological criteria for Ae. aegypti and photographed the mosquitoes. Entomologists from ARS Guadeloupe, ARS Martinique and from local research institutions examined the photographs and confirmed the agents’ suspicion that the mosquitoes were Ae. albopictus.
Two weeks later, an exploration mission was organized, during which agents from ARS Guadeloupe, ARS Saint Barthélémy, and the Institut Pasteur of Guadeloupe (IPG) traveled to Saint Barthélémy to confirm the species’ identity and to inform about its distribution area on the island. Larval prospections conducted from 14 to 16 October identified five breeding sites within a 400-m radius of the initial detection site, all containing mosquitoes morphologically identified as Ae. albopictus (Table 1).
Morphological criteria described by Darsie [5] were initially used during the missions to identify Ae. albopictus at both the larval and adult stages (Fig. 1a–c). However, since Ae. albopictus shares morphological similarities with other members of the Scutellaris group, further identification steps were performed on two collected specimens using matrix-assisted laser desorption ionization—time-of-flight mass spectrometry (MALDI-TOF MS) and cytochrome c oxidase 1 (cox1) gene barcoding to confirm the species. Briefly, the two specimens were individually dissected to separate the head and thorax from the rest of the body, and then homogenized 3 × 1 min at 30 Hz in a tissue lyzer (Retsch GmbH, Haan Germany). Legs and/or thoraxes were separately treated for identification by MALDI-TOF MS as previously described [6]. Protein mass profiles were acquired using a Maldi Biotyper Sirius Mass Spectrometer (Bruker Daltonics, Bremen, Germany), operating in linear positive-ion mode, with detection at a laser frequency of 50 Hz within a mass range of 2–20 kDa. MALDI-TOF spectra databases shared between entomologists from the Institut Pasteur of New Caledonia and the IHU Méditerranée enabled almost instantaneously confirmation of the samples as Ae. albopictus species, with the relevant score for the two specimens (log-score identification value = 2.05, matching spectra of Ae. albopictus from Cameroon [using mosquito legs from specimen 1] and from Marseille [using mosquito thorax from specimen 2]). To evaluate spectral similarity between specimen 1, which was collected in Saint-Barthélemy, and those from other Ae. albopictus, we generated a clustering analysis via the MSP (main spectra library) dendrogram function in MALDI-Biotyper v3.0 software. (Bruker Daltonics) using MS spectra from Ae. albopictus and five other Aedes species from the Scutellaris group (Aedes scutellaris, Aedes pseudoscutellaris, Aedes futunae, Aedes malayensis and Aedes polynesiensis). The resulting MSP dendrogram showed that the spectrum of the Saint-Barthélemy specimen clustered together with those of other Ae. albopictus, ruling out the possibility of other species from the Scutellaris group (Fig. 1d). These results highlighted the high reproducibility and the specificity of protein profiles among Aedes species, allowing a rapid and relevant identification of the specimen.
Identification of adult Aedes albopictus specimens found in Saint Barthélémy (French West Indies) according to morphological criteria (A, B, C), MALDI-TOF MS analysis (D) and cox1 gene barcoding (E). A Abdomen view: abdominal terga with complete basal white bands (red arrow), B thorax view: scutum with a medial-longitudinal white stripe (red arrow), C thorax view: mesepimeron with non-separated white scales, forming a V-shaped white spot (red arrow). D Main spectrum profile (MSP) dendrogram of Aedes Scutellaris Group. Spectrum from the mosquito specimen collected in Saint Barthélémy was obtained with a Maldi Biotyper Sirius mass spectrometer (Bruker Daltonics), and the dendrogram was generated by MALDI Biotyper Compas Explorer software. Distance units correspond to the relative similarity between spectra of Ae. albopictus from Saint Barthélémy and those from IHU Méditérranée and Institut Pasteur New Caledonia databases. E Molecular phylogenetic tree generated by the maximum likelihood method. The percentage bootstrap values shown at the nodes were calculated with 1000 replicates, and only bootstrap values > 70 are shown. Scale bar indicates nucleotide substitutions per site. Aedes albopictus sequences or spectra from Saint Barthelemy specimens are indicated in red and bold. cox1, Cytochrome c oxidase 1 gene; IHU, Institut hospitalo-universitaire; MALDI-TOF MS, matrix-assisted laser desorption ionization—time-of-flight mass spectrometry. Mosquito pictures were taken by Fabrice Sonor
We subsequently used the remaining body parts from the two specimens to conduct cox1 barcoding. Total DNA was extracted using the cetyltrimethylammonium bromide technique as previously described [7] and sequenced by Eurofins Genomics Europe (Sanger sequencing) using the primers HCO2198 and LCO1490 [6]. Sequences were analyzed, and multiple sequence alignment (Clustal W) was conducted using BioEdit version 7.0.5.3 software (BioEdit, Manchester, UK) [8, 9]. A molecular phylogenetic tree was generated by the maximum likelihood method based on the Timura 3-parameter model (best-fit nucleotide substitution pattern determined according to the corrected Akaike Information Criterion) using MEGA (http://www.megasoftware.net) with a bootstrap of 1000 replications [10]. Barcoding results indicated that the sequences from the mosquitoes collected in Saint Barthélemy (GenBank accession no. PQ644904 and PQ644905) shared 100% homology with sequences from Ae. albopictus collected in Europe and the Americas (Fig. 1e).
In the following weeks, weekly entomological surveillance continued, and the number of Ae. albopictus-positive breeding sites on Lorient increased to 18 in a 665-m2 area. The species’ presence was also detected in five breeding sites within a 150-m2 area in Saint Jean (17°53′50″N, 62°50′14″W). The discovery of this second hotspot, located 1.6 km from Lorient and near the airport, suggests a broader distribution of the species on the island. The presence of this invasive mosquito species in Saint Barthélémy, an island with daily aerial or maritime connections to the French Departments of the Americas [11], raises concerns about the risk of its introduction into these territories, as well as into other Caribbean countries. After the first detection of Ae. albopictus in the Dominican Republic in 1993 [4], the species has been reported in an increasing number of Caribbean territories, including Cuba in 1995 [12], Cayman Islands in 1997 [13, 14], Trinidad in 2002 [15], Haiti in 2010 [16], and Jamaica in 2018 [17] (Fig. 2).
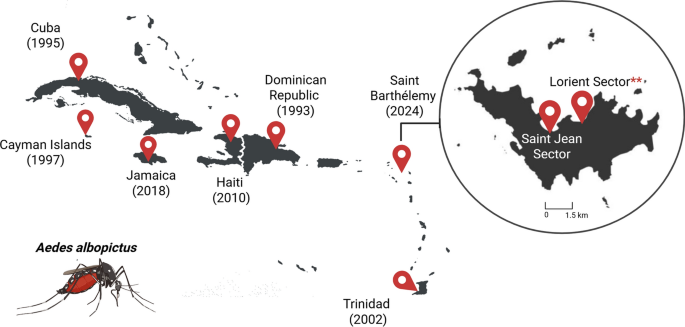
Aedes albopictus distribution in the Caribbean. Countries or territories reporting the species have been marked with red location pins. Date of first detection is given in parentheses. Asterisks indicate the first detection in Saint Barthélemy. cox1, Cytochrome c oxidase 1 gene; IHU, Institut hospitalo-universitaire; MALDI-TOF MS, matrix-assisted laser desorption ionization—time-of-flight mass spectrometry. Created with BioRender.com
It is noteworthy that in Cuba, from 1995 to 1999, Ae. albopictus was mainly distributed in peripheral municipalities with abundant vegetation rather that in more urbanized areas (i.e. city center). However, an increase in the species’ distribution in more urbanized areas, in association with Ae. aegypti, was observed from 2010 to 2018, highlighting the competitiveness of Ae. albopictus in the Caribbean context and an invasion process that can take several years [18]. In Saint Barthélemy, a 25-km2 island, Ae. albopictus is already present in artificial breeding sites from urban settings. In addition, half of these breeding sites contained the species in association with Ae. aegypti individuals (Table 1). In this context, the ecological plasticity of Ae. albopictus [19, 20] will likely facilitate its rapid invasion of Saint Barthélémy, emphasizing the urgent need to locally reinforce vector surveillance and control measures to prevent the further spread of this mosquito.
This work also highlights the efficiency of MALDI-TOF MS for rapidly identifying mosquito specimens in a real-life surveillance situation. The value of this approach for entomological surveillance has been repeatedly shown [21, 22]. Although MALDI-TOF MS-based identification requires reference spectra databases to identify specimens, as well as standardized protocols for spectrum acquisition [23], the exchange of databases between regions and institutes with access to different mosquito species represents a key development perspective. This is particularly relevant in our increasingly globalized world, where the potential for transcontinental movements of viruses and vectors continues to grow.