In recent years, a burgeoning legal operations discipline has helped corporate law departments enable the business more than ever before; but due to a lack of service-centric metrics, many don’t know how well they’re accomplishing that goal
Key takeaways:
-
-
-
Cost centers to strategic business enablers — In-house legal departments and general counsel in particular are increasingly focused on aligning their goals with broader company objectives, moving beyond traditional cost containment to emphasize service enhancement and business growth.
-
Current success metrics are still heavily focused on spend — Although GCs and legal operations professionals want to prioritize service and enable their businesses, most law departments continue to track and report metrics related primarily to costs and spending.
-
Updated metrics are essential for future success — To truly support business objectives, law departments must evolve their metrics and implement new data strategies to better capture service quality, business impact, and enablement.
In recent years, and especially in the AI age, corporate law departments have been tasked to increase efficiency, doing more with less and contributing back to the business at large. This has contributed to the rise of corporate legal operations as a discipline — what was fairly recently a niche concept for only the largest companies has morphed into a regular part of the corporate legal equation, and one that is increasingly tasked with keeping up with a burgeoning technology ecosystem and aligning the in-house legal function with larger company goals.
Increasingly, corporate law departments are fully embracing their role in enabling larger business objectives, and general counsel in particular are more focused than ever before on service enhancement for the business, according the 2025 Legal Department Operations (LDO) Index, published by the Thomson Reuters Institute (TRI) in conjunction with Buying Legal Council.
“Team mission: We are a trusted partner and strategic enabler, empowering [our company] and its builders to innovate with confidence,” answered one technology company GC about their team goal for legal operations. “We provide clear, practical legal guidance that removes friction and unlocks opportunity — driven by thoughtful leadership, collaboration, and operational excellence. The legal team intentionally tracks its greater vision to be the same as our company-wide mission.”
However, are law departments actually reaching that goal? That answer becomes a bit murkier, not the least of which because corporate law departments aren’t actually measuring what they identify as their goals. While GCs and corporate legal departments are gradually moving away from cost containment as their top priority, the primary success metrics they track by and large all deal with legal spend and spending impacts.
The message is clear: Law departments want to move away from being a cost center, but in order to truly accomplish that goal, they need to update their metrics and data gathering to actually move more in line with the business.
Aligning goals and metrics
Recent TRI research categorizes in-house legal departments’ role into four distinct categories: effectiveness, efficiency, protecting the business, and enabling growth. Each of these should be a focus of the department, but historically not all four have been given equal time. Many business leaders, for instance, traditionally have believed the legal department’s role to be more centered on protecting the business rather than enabling growth, leaving key business enablement to other internal teams in the company.
That shift is slowly changing, however. GCs are increasingly focused on aligning the department’s business goals with that of the larger organization, shifting the department from a cost center to a business generator, the LDO Index shows.
This is particularly true when comparing GC survey respondents with those respondents who hold a legal operations title. Among GC respondents, 47% say they are more focused on service enhancement than cost reduction, while just 7% say they are more focused on cost reduction. For legal ops professionals, that sentiment shifts, with a higher proportion focused on cost reduction (22%) and fewer focused on service enhancement (36%). This tracks with what is asked of each role, as legal operations professionals are typically tasked with more efficiency-centric and technology tasks, but GCs are conduits to the larger business.
Overall, however, both sides agree on one aspect: The legal department is shifting to become more service-focused than being simply about cost savings. It is interesting, then, that when asked what metrics are routinely reported on in their legal department, spend still remains far and away the top one. This is particularly surprising given that GCs are often the ones establishing these metrics, even though they hold a stronger stance towards service enablement than most legal operations professionals.
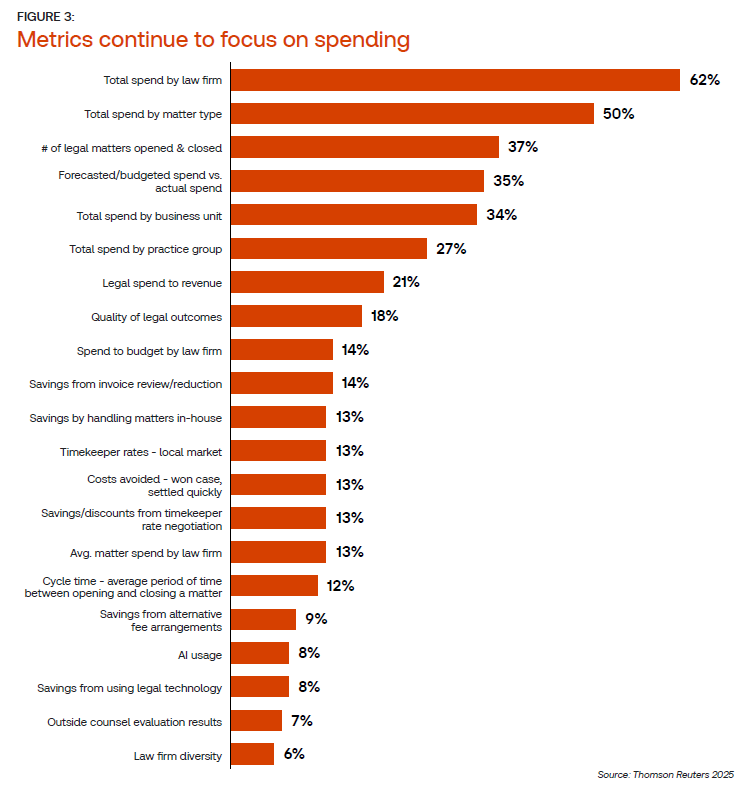
Indeed, spend by law firm and spend by matter type clearly dominate available metrics across many corporate law departments. Further, even many of the next most commonly tracked metrics available, according to one-third of respondents — forecasted versus actual spend, total spend by business unit, and total spend by practice group — still center around costs. Many of the service-centric metrics — such as quality of legal outcomes, cycle time, and costs avoided — are captured by less than 20% of respondent legal departments, the survey shows.
This also brings up one note about the survey: Respondents to this question leading to the chart above were given a choice from a list of predetermined metrics, meaning that conceptually, there may be service-centric metrics in use that are not listed here. However, additional open-ended research found in Thomson Reuters Market Insights backs up the assertion that many in-house legal departments are still primarily measuring costs, despite wishing to focus their overall priorities elsewhere.
Moving to better metrics tracking
There is recognition that the law department needs to evolve in order to serve constantly shifting business needs. And for many departments, meeting these needs starts by developing data sets that can be shared across the organization.
“I see my role evolving into a more strategic, innovation-focused function — leading digital transformation, leveraging [generative] AI for smarter legal service delivery, and aligning legal operations with enterprise-wide goals,” said one energy industry legal operations professional. “I anticipate deeper collaboration across departments, greater emphasis on data analytics, and a continued shift from process execution to proactive business enablement.”
In fact, many respondents — particularly those at companies with newer legal operations functions — did note that better metrics tracking is on their roadmap for the future. And some said their primary goal at first was simply to become established, and more business-centric goals would come next.
“We have just started to develop the legal operations function at the company over the past year and a half,” said one financial industry legal operations professional when asked how they see their role evolving. “During the first part of this second year, we have been focused on legal spend and tracking our work with outside counsel to see where we can reduce our overall spend and ensure we are receiving the best service from our outside counsel. The second half will be focused on furthering our knowledge management base internally with shared resources and regular tracking of regulatory risks… so as to better be able to support the business.”
Yet, this focus is not universal, and especially for law departments that have not updated their data gathering and metrics capabilities, the time for thoughtful action is now. The LDO Index concludes with 10 practical actions that legal department leaders can undertake, with one of them explicitly calling to implement key metrics for data-driven decision making. This goal should be a top priority for all legal departments, regardless of whether the action comes from a GC or a professional with a background in legal operations.
Entering 2026, GCs and legal operations professionals alike should look into supercharging their metrics for success, focusing on how they can better measure business enablement and promote service enhancement more than ever before.