FOR IMMEDIATE RELEASE:
Journal Name: Cell Reports
Pub Date: August 12th
Title of the Article Clonotype-Enriched Somatic Hypermutations Drive Affinity Maturation of a Public Human Antibody Targeting an Occluded Sarbecovirus Epitope
Corresponding Author: Camila Coelho, PhD, Assistant Professor of Microbiology, Icahn School of Medicine at Mount Sinai
Bottom Line: Multiple humans exposed to SARS-CoV-2 elicit an antibody called M15. This antibody has a rare ability to bind a previously unknown target in the spike protein of the virus by undergoing rare mutations that are highly specific to this antibody class as compared to similar antibodies targeting other pathogens.
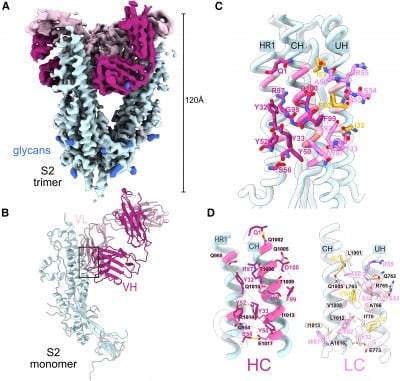
Results: M15, a public antibody against SAS-CoV-2, undergoes unique mutations in its light chain that are common in different people that elicit this antibody upon infection or vaccination. These mutations help M15 gain access to and bind stronger (increase affinity) to a newly discovered target (or epitope) on the spike protein of SARS-CoV-2. Interestingly, the blood level of antibodies targeting this region increases with multiple vaccine doses.
Why the Research Is Interesting: The findings identify features that differentiate mutations improving the affinity of an antibody and also uncover a novel epitope on the spike protein of SARS-CoV-2.
Who: Humans who have been vaccinated or infected with SARS-CoV-2.
When: Days to Years post SARS-CoV-2-infection or vaccination.
What: SARS-CoV-2 infected or vaccinated individuals elicit a public antibody that targets a novel epitope, assisted by rare antibody mutations that are almost exclusively seen in these antibodies.
How: M15-like antibodies were specifically identified in SARS-CoV-2 infected and vaccinated individuals based on sequence identity. Biolayer interferometry, that measures the affinity of an antibody to its epitope, coupled with a cryo-electron microscopy structure of the M15 molecule in complex with SARS-CoV-2, were used to investigate the role of convergent and clonotype-enriched mutations in improving the affinity of M15. Serum competition ELISA was used to measure the level of antibodies in blood targeting this epitope post infection or vaccination.
Study Conclusions: Mutations in the light chain that improve affinity of a public antibody, M15, against SARS-CoV-2 are convergently acquired in multiple individuals and are enriched in the public antibody class compared to antibodies recognizing other pathogens. Such mutations help M15 access a highly conserved, novel epitope on the spike protein of SARS-CoV-2. Levels of serum antibodies targeting this viral region increase upon multiple COVID-19 vaccinations.
Said Mount Sinai’s Dr. Camila Coelho of the research: “Our study shows that certain patterns in antibody mutations, such as clonotype-enrichment and convergence, can help identify which mutations actually make antibodies stronger with respect to binding. This could also be applicable to other diseases, not just COVID-19. And that’s why Mount Sinai’s PhD student Vishal Rao is further investigating this phenomenon in our laboratory. It’s a step toward designing smarter vaccines that fine-tune the immune system to produce more potent antibodies.”
To request a copy of the paper or to schedule an interview with Dr. Coelho, please contact Mount Sinai’s Director of Media and Public Affairs, Elizabeth Dowling, at [email protected] or at 347-541-0212.