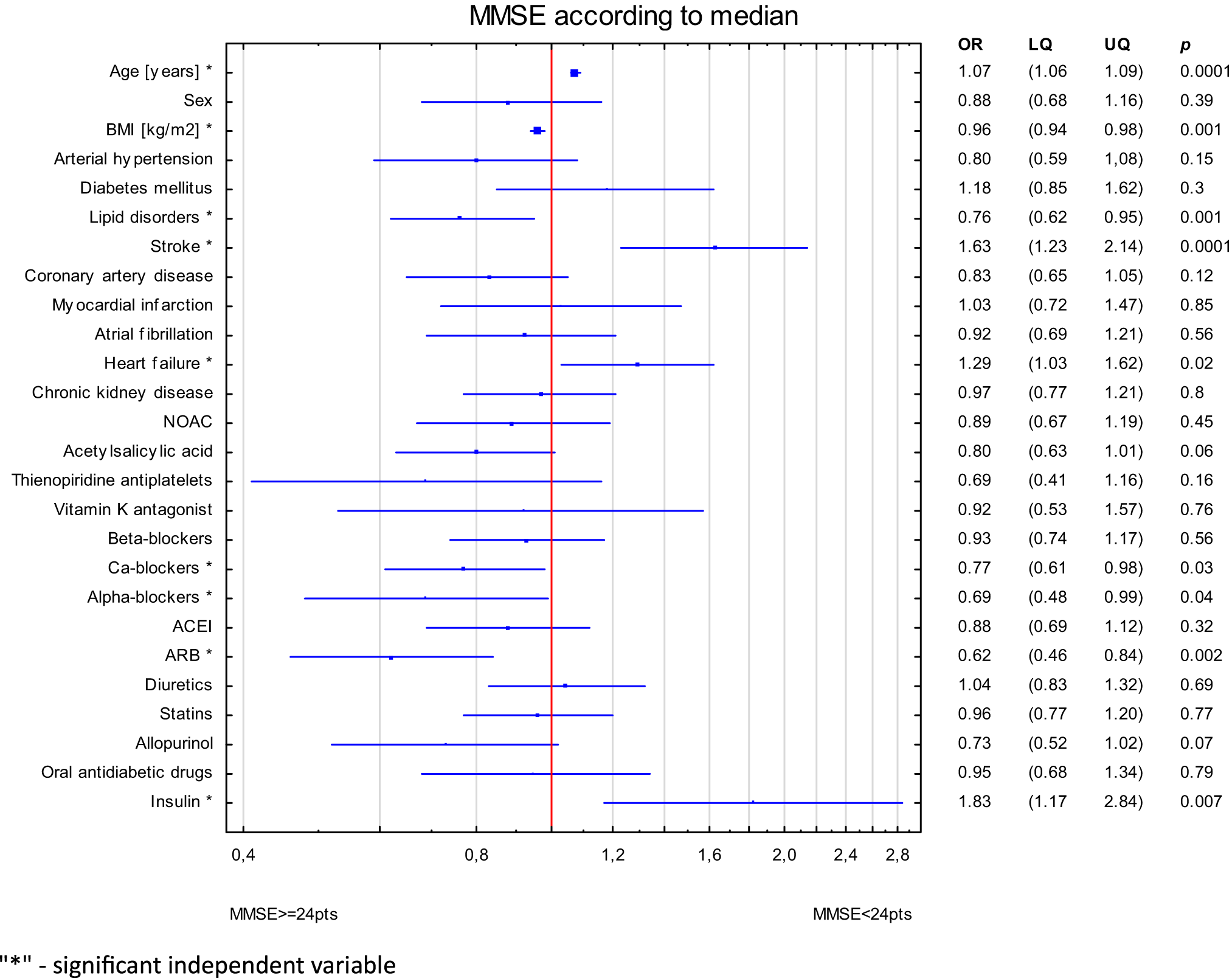
The study aims to explore the association between cardiometabolic diseases, their corresponding medications, and cognitive function in older patients. Our findings indicate that health conditions and prescribed drugs appear to be linked to cognition, with associations observed in both higher and diminished cognitive functioning.
In our study, logistic regression analysis reveals that lipid disorders and BMI are linked to normal MMSE. Conversely, age and previous stroke are associated with the symptoms of dementia. Relation of heart failure was of borderline significance. Regarding pharmacotherapy, the independent association with higher MMSE values is observed with the use of CCBs, alpha-blockers and ARBs. However, insulin intake demonstrates a connection with cognitive decline.
The cognitive function assessed through the prism of cardiometabolic disorders seems intertwined. BMI correlates positively with MMSE value, indicating that higher BMI is associated with normal cognitive function. Cao et al. indicates that BMI is independent predictors of dementia, but the study concerned people aged 37–73 [33]. The relationship between BMI, age and cognitive function appears to be age-dependent. Higher BMI is associated with symptoms of dementia in middle age, while among seniors, higher BMI is linked with better cognition and decreased mortality [34]. Finally, the study in the group of two million people indicates that low body mass in middle age and old age increases the risk of dementia, whereas obesity lowers it by 29% [35]. Hypertension and past strokes are attributed to VaD [36,37,38] Elevated blood pressure leads to microcirculation disorders and neuronal damage [39]. Another disorder associated with hypertension is AD, as high blood pressure is linked with the accumulation of Aβ and TAU protein. Some researchers suggest that the pathophysiological changes in VaD and AD overlap [21, 40]. Hence, the presence of HA is proven to be strictly linked not only with VaD, but also AD, or MCI [21, 41, 42]. Lipid disorders, are closely associated with dementia risk [22, 43]. In our research, we found that lipid disorders were associated with reduced risk of cognitive decline. This finding was initially unexpected and contradicting to initial beliefs. However, a more thorough examination revealed that individuals with lipid disorders were notably younger than their counterparts without such conditions. Another explanation may be associated with abovementioned protective effect of higher BMI, with lipid disorders claimed as its derivative.
The Framingham post-stroke subjects had a 2.0–2.8 times higher risk of dementia compared to healthy individuals [21]. The interaction between these conditions is not limited to the loss of neurons and memory impairment [38, 44]. The connection between ischemic stroke and cerebral dementia lies in vascular and neurodegenerative processes [45,46,47] like the development of neurofibrillary tangles and intraneural plaques [48]. Furthermore, the accumulated Aβ in blood vessels damages the blood-brain barrier, and impaired clearance mechanisms play critical role in the development of post-stroke dementia [37, 49, 50]. Similarmechanisms may explain the relationship between heart disease and dementia. Coronary artery disease, myocardial infarction, atrial fibrillation, valvular disease, or heart failure cause hemodynamic stress, reduced blood flow to the brain, neuroinflammation, and increased coagulation what substantially elevate the dementia risk [6,7,8]. Furthermore, different heart failure types correlate with varied risks of developing specific dementia types, impacting patient survival rates [51]. Besides, cardiac dysfunction-induced reduction in cerebral blood flow may intensify the pathology of VaD and AD [52]. Presented study may confirm some of the mentioned associations.
Primary risk factors like BMI or lipid disorders were associated with significantly better cognitive status. They are related to younger age and their meaning seems different in advanced-age population. Arterial hypertension may be considered as intermediate factor – both disease and risk factor for cardiometabolic diseases and dementia. Stroke and heart failure results from lifetime accumulation of risk factors, are typical conditions of advanced age and were independently associated with poorer cognitive performance. In this context our results may support the hypothesis that dementia may be strongly associated with cardiometabolic risk.
Another aspect was to investigate the relationship between cardiometabolic pharmacotherapy and cognitive performance. Interesting research highlights the link between low-dose ASA use and a lower incidence of dementia in individuals with chronic heart conditions, showing a 31% decrease in AD risk, a 69% decrease in VaD risk, and a 34% decrease in overall dementia risk after a minimum of 10 years of medication use [53]. Other studies present mixed findings, indicating that aspirin might lower AD risk in older patients with ischemic stroke [54], or finding no significant link between aspirin use and dementia risk [55, 56]. The precise mechanisms through which ASA may trigger neuroprotection, such as its anti-inflammatory and anticoagulant properties potentially reducing cerebral micro-infarcts, remain only partially understood.
The knowledge for hypotensive agents is even more complex. Several studies suggest that the impact of antihypertensive medications on lowering the risk of dementia is associated with substances that help to maintain regular cerebral blood flow, such as CCBs, ACE inhibitors, and ARBs. This observation may clarify the absence of a similar effect in the case of certain medications, including diuretics [21].The findings on the connection between CCBs and dementia are conflicting, indicating that some of CCBs may help prevent cognitive deterioration [21, 57] or showing no effect [58]. Molecularly, elevated intracellular calcium can trigger neuronal death, activation of enzymes that degrade cellular components, production of reactive oxygen species, and the initiation of apoptosis [59]. Aβ in oligomeric state, play a role in the progression of AD by creating pores in the membranes of neurons, leading to excessive calcium entry, neuronal damage, oxidative stress, neuroinflammation and ultimately apoptosis. CCBs potentially reduce this adverse effect [60]. The research about alpha-blockers indicate on substance depending positive or negative effect on dementia risk [61], pointing on tamsulosin as a substance with elevated risk of cognitive decline [62] and doxazosin carrying neuroprotective effect [63]. ARBs may also offer protective effect against dementia. The OSCAR study, which involved more than 25,000 participants aged 50 and above, showed a distinct adverse relationship between eplenosartan intake and cognitive deterioration. Additionally, the study describing comparison between the impact of an ACE inhibitor plus diuretic versus an ARB plus diuretic, proved better cognitive functions with the use of the sartan-included course [21]. ARBs, by inhibiting the angiotensin II type 1 receptor, reduce blood pressure, but also reduce inflammation, improve endothelial function, and decrease oxidative stress, all of which could indirectly impact the mechanisms involved in the onset of dementia [64]. ARBs could influence against cognitive deterioration, potentially due to their capacity to penetrate the blood-brain barrier and directly influence brain tissue [64].
The impact of statins on dementia remains disputable with conflicting evidence, with studies suggesting both neuroprotective and neurodegenerative effects from these medications [65]. Insulin plays crucial roles in the brain, including learning and memory processes, which are disturbed in AD. Brain insulin resistance emerges as a pathological hallmark of AD, leading to dysregulation in metabolism, inflammation, oxidative stress, impaired insulin signalling, mitochondrial dysfunction and synaptic dysfunction [66,67,68]. These conditions act as molecular links between diabetes and AD. In both disorders, constant inflammation induces insulin resistance, what next exacerbates neurodegeneration and cognitive decline. Targeting inflammation, insulin resistance and insulin’s role in the metabolism of Aβ and the phosphorylation of tau, essential in AD’s pathology present a promising direction for novel AD therapies [66, 69, 70]. Our study shows that there is both a positive and negative relationship between cardiometabolic drugs and cognitive performance. CCBs, alpha-blockers and ARBs appear to be associated with better cognitive performance. Furthermore, the association of ARBs and cognitive function may designate on that group as the strongest protective effect among the various studied drug groups. Otherwise, the relation of insulin and worse cognitive performance may highlight the significance of metabolic pathways in the development of dementia. In our study, insulin was used in younger subjects indicating on long-term accumulation of cardiometabolic risk.
An important consideration in interpreting our findings is the broader geriatric context, particularly the roles of frailty, polypharmacy, and antidepressants use. Frailty, although not directly assessed in our study, is closely linked to both cardiovascular disease and cognitive decline and may influence the observed associations [71]. Similarly, while polypharmacy was common in our cohort, it was not independently associated with worse cognitive outcomes [72], though its cumulative effects may still be clinically relevant. Lastly, the lack of data on antidepressants use—despite its known impact on cognition in older adults [73]—represents a limitation and a potential confounder that should be addressed in future research.
A key strength of our study lies in its comprehensive coverage of cardiometabolic diseases, and the extensive variety of medications tested in relation to dementia. It’s also significant to mention that our research involves a notably large group of older individuals. Nonetheless, there are several limitations to our study that need attention.
This study’s cross-sectional, retrospective and observational design prevents establishing causal relationships between cardiometabolic factors, medications, and cognitive outcomes. The sample was drawn from a single geriatric hospital in Central Poland, which may limit the generalizability of findings to broader or more diverse populations. Furthermore, by focusing primarily on cardiometabolic variables, other potential contributors to cognitive decline, such as genetic, psychosocial, or lifestyle factors may not have been fully accounted for. Additionally, the study did not account for the duration of medication use, as data were limited to current prescriptions at the time of hospitalization; this may have influenced the observed associations between pharmacotherapy and cognitive function.
To address these scarcities, future prospective studies should be larger in scale, multicentric, and conducted across varied demographic groups. That would facilitate detailed and extensive conclusions.