Flemming H-C, Wingender J, Szewzyk U, Steinberg P, Rice SA, Kjelleberg S. Biofilms: an emergent form of bacterial life. Nat Rev Microbiol. 2016;14(9):563–75.
Google Scholar
Zuberi A, Ahmad N, Ahmad H, Saeed M, Ahmad I. Beyond antibiotics: CRISPR/Cas9 triumph over biofilm-associated antibiotic resistance infections. Front Cell Infect Microbiol. 2024;14:1408569.
Google Scholar
Ciofu O, Tolker-Nielsen T. Tolerance and resistance of Pseudomonas aeruginosa biofilms to antimicrobial agents-how P. aeruginosa can escape antibiotics. Front Microbiol. 2019;10:913.
Google Scholar
Jinek M, Chylinski K, Fonfara I, Hauer M, Doudna JA, Charpentier E. A programmable dual-RNA–guided DNA endonuclease in adaptive bacterial immunity. Science. 2012;337(6096):816–21.
Google Scholar
Ekwebelem OC, Aleke J, Ofielu E, Nnorom-Dike O. CRISPR-Cas9 system: a revolutionary tool in the fight against antimicrobial resistance: retracted. Infect Microbes Dis. 2021;3(2):51–6.
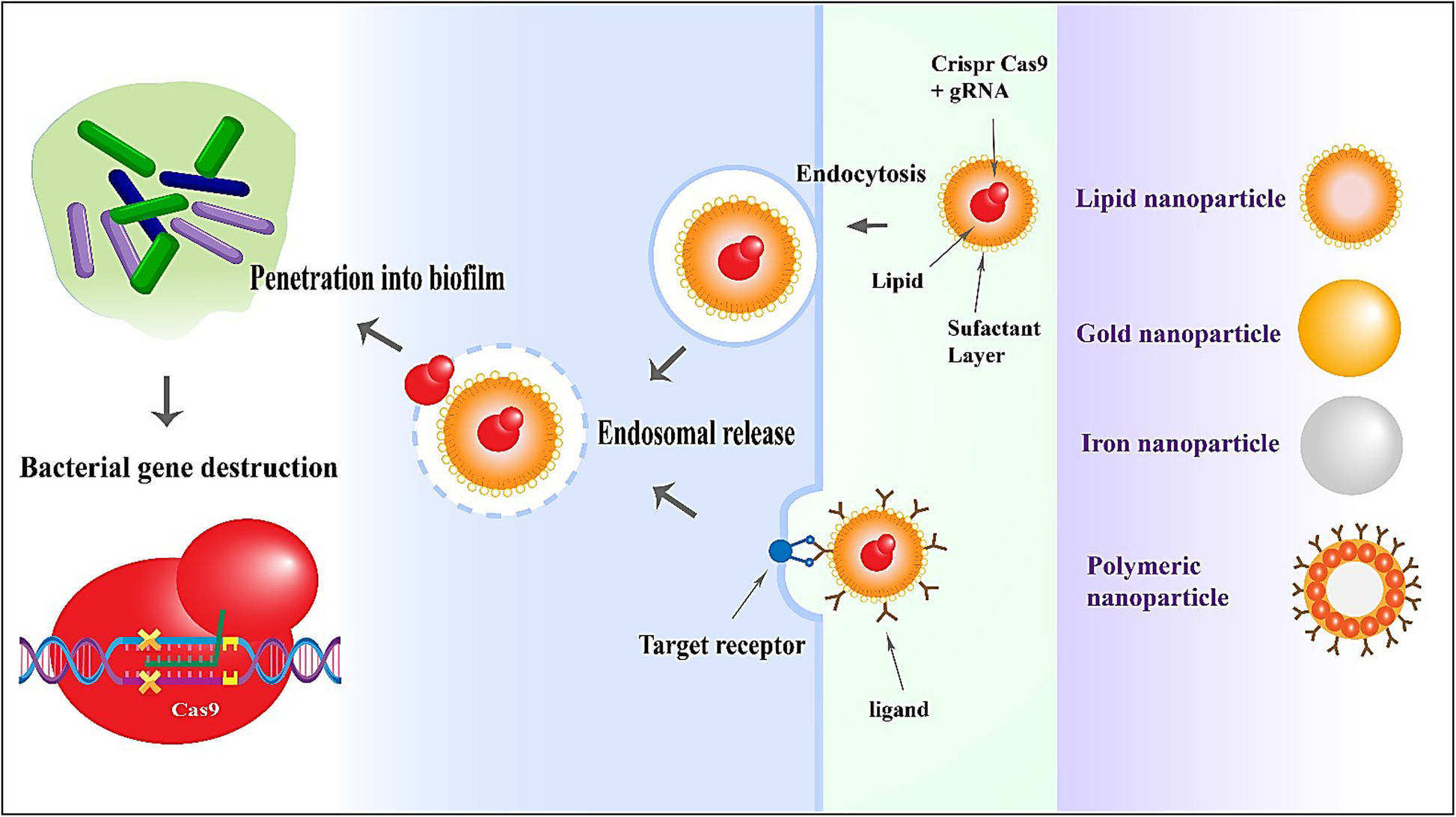
Fletcher RB, Stokes LD, Kelly IB 3rd, Henderson KM, Vallecillo-Viejo IC, Colazo JM, et al. Nonviral in vivo delivery of CRISPR-Cas9 using protein-agnostic, high-loading porous silicon and polymer nanoparticles. ACS Nano. 2023;17(17):16412–31.
Google Scholar
Wan F, Draz MS, Gu M, Yu W, Ruan Z, Luo Q. Novel strategy to combat antibiotic resistance: a sight into the combination of CRISPR/Cas9 and nanoparticles. Pharmaceutics. 2021;13(3):352.
Google Scholar
Zohra T, Numan M, Ikram A, Salman M, Khan T, Din M, et al. Cracking the challenge of antimicrobial drug resistance with CRISPR/Cas9, nanotechnology and other strategies in ESKAPE pathogens. Microorganisms. 2021;9(5):954.
Google Scholar
Kim M, Hwang Y, Lim S, Jang H-K, Kim H-O. Advances in nanoparticles as non-viral vectors for efficient delivery of CRISPR/Cas9. Pharmaceutics. 2024;16(9):1197.
Google Scholar
Gold K, Slay B, Knackstedt M, Gaharwar AK. Antimicrobial activity of metal and metal-oxide based nanoparticles. Adv Ther. 2018;1(3):1700033.
Jiang Y, Wu R, Zhang W, Xin F, Jiang M. Construction of stable microbial consortia for effective biochemical synthesis. Trends Biotechnol. 2023;41(11):1430–41.
Google Scholar
Bush K, Bradford PA. β-lactams and β-lactamase inhibitors: an overview. Cold Spring Harb Perspect Med. 2016;6(8):a025247.
Google Scholar
Davies J, Davies D. Origins and evolution of antibiotic resistance. Microbiol Mol Biol Rev. 2010;74(3):417–33.
Google Scholar
Hay SI, Rao PC, Dolecek C, Day NP, Stergachis A, Lopez AD, Murray CJ: Measuring and mapping the global burden of antimicrobial resistance. BMC medicine 2018, 16(1):78.
Google Scholar
Delcour AH. Outer membrane permeability and antibiotic resistance. Biochim Biophys Acta. 2009;1794(5):808–16.
Google Scholar
Laxminarayan R, Duse A, Wattal C, Zaidi AK, Wertheim HF, Sumpradit N, et al. Antibiotic resistance—the need for global solutions. Lancet Infect Dis. 2013;13(12):1057–98.
Google Scholar
van Belkum A, Soriaga LB, LaFave MC, Akella S, Veyrieras J-B, Barbu EM, et al. Phylogenetic distribution of CRISPR-Cas systems in antibiotic-resistant Pseudomonas aeruginosa. MBio. 2015;6(6). https://doi.org/10.1128/mBio.01796-15.
Allegranzi B, Gayet-Ageron A, Damani N, Bengaly L, McLaws M-L, Moro M-L, et al. Global implementation of WHO’s multimodal strategy for improvement of hand hygiene: a quasi-experimental study. Lancet Infect Dis. 2013;13(10):843–51.
Google Scholar
Tängdén T, Giske C. Global dissemination of extensively drug-resistant carbapenemase-producing E nterobacteriaceae: clinical perspectives on detection, treatment and infection control. J Intern Med. 2015;277(5):501–12.
Google Scholar
Laxminarayan R, Impalli I, Rangarajan R, Cohn J, Ramjeet K, Trainor BW, Strathdee S, Sumpradit N, Berman D, Wertheim H: Expanding antibiotic, vaccine, and diagnostics development and access to tackle antimicrobial resistance. The Lancet 2024;403(10443):2534–2550.
Spellberg B, Guidos R, Gilbert D, Bradley J, Boucher HW, Scheld WM, et al. The epidemic of antibiotic-resistant infections: a call to action for the medical community from the Infectious Diseases Society of America. Clin Infect Dis. 2008;46(2):155–64.
Google Scholar
Dyar O, Pagani L, Pulcini C. Strategies and challenges of antimicrobial stewardship in long-term care facilities. Clin Microbiol Infect. 2015;21(1):10–9.
Google Scholar
Coates AR, Halls G, Hu Y. Novel classes of antibiotics or more of the same? Br J Pharmacol. 2011;163(1):184–94.
Google Scholar
Pandey P, Sirisha VL. From gene editing to biofilm busting: CRISPR-CAS9 against antibiotic resistance—a review. Cell Biochem Biophys. 2024;82:1–12.
Juszczuk-Kubiak E. Molecular aspects of the functioning of pathogenic bacteria biofilm based on quorum sensing (QS) signal-response system and innovative non-antibiotic strategies for their elimination. Int J Mol Sci. 2024;25(5):2655.
Google Scholar
Saharan B, Beniwal N, Duhan J. From formulation to function: a detailed review of microbial biofilms and their polymer-based extracellular substances. The Microbe. 2024;5:100194.
Costerton JW, Stewart PS, Greenberg EP. Bacterial biofilms: a common cause of persistent infections. Science. 1999;284(5418):1318–22.
Google Scholar
Nichols W. Biofilms, antibiotics and penetration. Rev Med Microbiol. 1991;2:177–81.
Xie Y, Liu H, Teng Z, Ma J, Liu G. Nanomaterial-enabled anti-biofilm strategies: new opportunities for treatment of bacterial infections. Nanoscale. 2025;17(10):5605–28.
Google Scholar
Domenech M, García E, Moscoso M. Biofilm formation in Streptococcus pneumoniae. Microb Biotechnol. 2012;5(4):455–65.
Google Scholar
Balcázar JL, Subirats J, Borrego CM. The role of biofilms as environmental reservoirs of antibiotic resistance. Front Microbiol. 2015;6:1216.
Google Scholar
Keren I, Kaldalu N, Spoering A, Wang Y, Lewis K. Persister cells and tolerance to antimicrobials. FEMS Microbiol Lett. 2004;230(1):13–8.
Google Scholar
Já K. Biofilm dispersal: mechanisms, clinical implications, and potential therapeutic uses. J Dent Res. 2010;89(3):205–18.
Patriquin GM, Banin E, Gilmour C, Tuchman R, Greenberg EP, Poole K. Influence of quorum sensing and iron on twitching motility and biofilm formation in Pseudomonas aeruginosa. J Bacteriol. 2008;190(2):662–71.
Google Scholar
Abebe GM. The role of bacterial biofilm in antibiotic resistance and food contamination. Int J Microbiol. 2020;2020(1):1705814.
Google Scholar
Hancock RE, Speert DP. Antibiotic resistance in Pseudomonas aeruginosa: mechanisms and impact on treatment. Drug Resist Updat. 2000;3(4):247–55.
Google Scholar
Redfern J, Wallace J, van Belkum A, Jaillard M, Whittard E, Ragupathy R, et al. Biofilm associated genotypes of multiple antibiotic resistant Pseudomonas aeruginosa. BMC Genomics. 2021;22:1–16.
Luo Y, Yang Q, Zhang D, Yan W. Mechanisms and control strategies of antibiotic resistance in pathological biofilms. J Microbiol Biotechnol. 2020;31(1):1.
Google Scholar
Haddad Kashani H, Schmelcher M, Sabzalipoor H, Seyed Hosseini E, Moniri R: Recombinant endolysins as potential therapeutics against antibiotic-resistant Staphylococcus aureus: current status of research and novel delivery strategies. Clinical microbiology reviews 2018;31(1). https://doi.org/10.1128/cmr.00071-00017.
Kashani HH, Moniri R. Expression of recombinant pET22b-LysK-cysteine/histidine-dependent amidohydrolase/peptidase bacteriophage therapeutic protein in Escherichia coli BL21 (DE3). Osong Public Health Res Perspect. 2015;6(4):256–60.
Google Scholar
Hosseini ES, Moniri R, Goli YD, Kashani HH. Purification of antibacterial CHAPK protein using a self-cleaving fusion tag and its activity against methicillin-resistant Staphylococcus aureus. Probiotics Antimicrob Proteins. 2016;8(4):202–10.
Google Scholar
Van Hoogstraten S, Kuik C, Arts J, Cillero-Pastor B: Molecular imaging of bacterial biofilms—a systematic review. Critical reviews in microbiology 2024;50(6):971–992.
Hall CW, Mah T-F. Molecular mechanisms of biofilm-based antibiotic resistance and tolerance in pathogenic bacteria. FEMS Microbiol Rev. 2017;41(3):276–301.
Google Scholar
Li P, Wan P, Zhao R, Chen J, Li X, Li J, et al. Targeted elimination of bla NDM-5 gene in Escherichia coli by conjugative CRISPR-Cas9 system. Infect Drug Resist. 2022. https://doi.org/10.2147/IDR.S357470.
Google Scholar
Marraffini LA. CRISPR-Cas immunity in prokaryotes. Nature. 2015;526(7571):55–61.
Google Scholar
Vigouroux A, Bikard D. CRISPR tools to control gene expression in bacteria. Microbiol Mol Biol Rev. 2020;84(2). https://doi.org/10.1128/MMBR.00077-19.
Singh V, Gohil N, Ramirez Garcia R, Braddick D, Fofié CK. Recent advances in CRISPR-Cas9 genome editing technology for biological and biomedical investigations. J Cell Biochem. 2018;119(1):81–94.
Google Scholar
Sapranauskas R, Gasiunas G, Fremaux C, Barrangou R, Horvath P, Siksnys V. The Streptococcus thermophilus CRISPR/Cas system provides immunity in Escherichia coli. Nucleic Acids Res. 2011;39(21):9275–82.
Google Scholar
Wu Y, Battalapalli D, Hakeem MJ, Selamneni V, Zhang P, Draz MS, et al. Engineered CRISPR-Cas systems for the detection and control of antibiotic-resistant infections. J Nanobiotechnol. 2021;19:1–26.
Google Scholar
Zhang L, Wang L, Xie Y, Wang P, Deng S, Qin A, et al. Triple-targeting delivery of CRISPR/Cas9 to reduce the risk of cardiovascular diseases. Angew Chem Int Ed Engl. 2019;58(36):12404–8.
Google Scholar
Timin AS, Muslimov AR, Lepik KV, Epifanovskaya OS, Shakirova AI, Mock U, et al. Efficient gene editing via non-viral delivery of CRISPR-Cas9 system using polymeric and hybrid microcarriers. Nanomedicine. 2018;14(1):97–108.
Google Scholar
Gratacap RL, Regan T, Dehler CE, Martin SAM, Boudinot P, Collet B, et al. Efficient CRISPR/Cas9 genome editing in a salmonid fish cell line using a lentivirus delivery system. BMC Biotechnol. 2020;20(1):35.
Google Scholar
Glass Z, Li Y, Xu Q. Nanoparticles for CRISPR-Cas9 delivery. Nat Biomed Eng. 2017;1(11):854–5.
Google Scholar
Sago CD, Lokugamage MP, Paunovska K, Vanover DA, Monaco CM, Shah NN, et al. High-throughput in vivo screen of functional mRNA delivery identifies nanoparticles for endothelial cell gene editing. Proc Natl Acad Sci U S A. 2018;115(42):E9944–52.
Google Scholar
Zhang R, Xu W, Shao S, Wang Q. Gene silencing through CRISPR interference in bacteria: current advances and future prospects. Front Microbiol. 2021;12:635227.
Google Scholar
Lee B, Lee K, Panda S, Gonzales-Rojas R, Chong A, Bugay V, et al. Nanoparticle delivery of CRISPR into the brain rescues a mouse model of fragile X syndrome from exaggerated repetitive behaviours. Nat Biomed Eng. 2018;2(7):497–507.
Google Scholar
Liu Y, Zhao G, Xu CF, Luo YL, Lu ZD, Wang J. Systemic delivery of CRISPR/Cas9 with PEG-PLGA nanoparticles for chronic myeloid leukemia targeted therapy. Biomater Sci. 2018;6(6):1592–603.
Google Scholar
Shahbazi R, Sghia-Hughes G, Reid JL, Kubek S, Haworth KG, Humbert O, et al. Targeted homology-directed repair in blood stem and progenitor cells with CRISPR nanoformulations. Nat Mater. 2019;18(10):1124–32.
Google Scholar
Kadkhoda H, Gholizadeh P, Kafil HS, Ghotaslou R, Pirzadeh T, Rezaee MA, et al. Role of CRISPR-Cas systems and anti-CRISPR proteins in bacterial antibiotic resistance. Heliyon. 2024. https://doi.org/10.1016/j.heliyon.2024.e34692.
Google Scholar
Nath A, Bhattacharjee R, Nandi A, Sinha A, Kar S, Manoharan N, et al. Phage delivered CRISPR-Cas system to combat multidrug-resistant pathogens in gut microbiome. Biomed Pharmacother. 2022;151:113122.
Google Scholar
Wang H, La Russa M, Qi LS. CRISPR/Cas9 in genome editing and beyond. Annu Rev Biochem. 2016;85(1):227–64.
Google Scholar
Asmamaw M, Zawdie B. Mechanism and applications of CRISPR/Cas-9-mediated genome editing. Biologics. 2021;15:353–61.
Google Scholar
Barrangou R, Fremaux C, Deveau H, Richards M, Boyaval P, Moineau S, et al. CRISPR provides acquired resistance against viruses in prokaryotes. Science. 2007;315(5819):1709–12.
Google Scholar
Angel PASRY, Raghul M, Gowsalya S, Paulkumar K, Murugan K: CRISPR interference system: a potential strategy to inhibit pathogenic biofilm in the agri-food sector. In: CRISPR and RNAi Systems. edn.: Elsevier; 2021:387–403.
Kim J-S, Cho D-H, Park M, Chung W-J, Shin D, Ko KS, et al. CRISPR/Cas9-mediated re-sensitization of antibiotic-resistant Escherichia coli harboring extended-spectrum β-lactamases. J Microbiol Biotechnol. 2016;26(2):394–401.
Google Scholar
Tao S, Chen H, Li N, Liang W. The application of the CRISPR-Cas system in antibiotic resistance. Infect Drug Resist. 2022. https://doi.org/10.2147/IDR.S370869.
Google Scholar
Palacios Araya D, Palmer KL, Duerkop BA. Crispr-based antimicrobials to obstruct antibiotic-resistant and pathogenic bacteria. PLoS Pathog. 2021;17(7):e1009672.
Google Scholar
Luo M, Jia Y-Y, Jing Z-W, Li C, Zhou S-Y, Mei Q-B, et al. Construction and optimization of pH-sensitive nanoparticle delivery system containing PLGA and UCCs-2 for targeted treatment of Helicobacter pylori. Colloids Surf, B. 2018;164:11–9.
Google Scholar
Khambhati K, Bhattacharjee G, Gohil N, Dhanoa GK, Sagona AP, Mani I, et al. Phage engineering and phage-assisted CRISPR-Cas delivery to combat multidrug-resistant pathogens. Bioeng Transl Med. 2023;8(2):e10381.
Google Scholar
Gliźniewicz M, Miłek D, Olszewska P, Czajkowski A, Serwin N, Cecerska-Heryć E, et al. Advances in bacteriophage-mediated strategies for combating polymicrobial biofilms. Front Microbiol. 2024;14:1320345.
Google Scholar
Radovic-Moreno AF, Lu TK, Puscasu VA, Yoon CJ, Langer R, Farokhzad OC. Surface charge-switching polymeric nanoparticles for bacterial cell wall-targeted delivery of antibiotics. ACS Nano. 2012;6(5):4279–87.
Google Scholar
Zaman QU, Chu W, Hao M, Shi Y, Sun M, Sang S-F, et al. CRISPR/Cas9-mediated multiplex genome editing of JAGGED gene in Brassica napus L. Biomolecules. 2019;9(11):725.
Google Scholar
Bondy-Denomy J. Protein inhibitors of CRISPR-Cas9. ACS Chem Biol. 2018;13(2):417–23.
Google Scholar
Chabas H, Nicot A, Meaden S, Westra ER, Tremblay DM, Pradier L, et al. Variability in the durability of CRISPR-Cas immunity. Philos Trans R Soc Lond B Biol Sci. 2019;374(1772):20180097.
Google Scholar
Aslam B, Rasool M, Idris A, Muzammil S, Alvi RF, Khurshid M, et al. CRISPR-Cas system: a potential alternative tool to cope antibiotic resistance. Antimicrob Resist Infect Control. 2020;9:1–3.
Ortiz-Cartagena C, Fernández-Grela P, Armán L, Blasco L, Pablo-Marcos D, Bleriot I, Fernández-García L, Ibarguren-Quiles C, Fernández-Cuenca F, Barrio-Pujante A:.The LAMP-CRISPR-Cas13a technique for detecting the CBASS mechanism of phage resistance in bacteria. Frontiers in Microbiology. 2025;16:1550534.
Gupta A, Saleh NM, Das R, Landis RF, Bigdeli A, Motamedchaboki K, et al. Synergistic antimicrobial therapy using nanoparticles and antibiotics for the treatment of multidrug-resistant bacterial infection. Nano Futures. 2017;1(1):015004.
Fatima F, Siddiqui S, Khan WA. Nanoparticles as novel emerging therapeutic antibacterial agents in the antibiotics resistant era. Biol Trace Elem Res. 2021;199(7):2552–64.
Google Scholar
Mulens-Arias V, Rojas JM, Barber DF. The intrinsic biological identities of iron oxide nanoparticles and their coatings: unexplored territory for combinatorial therapies. Nanomaterials. 2020;10(5):837.
Google Scholar
Jacob EM, Borah A, Sakthi KD. CRISPR/Cas9 Nano-delivery Approaches for Targeted Gene Therapy. Pharmaceutical Nanobiotechnology for Targeted Therapy: Springer; 2022. p. 27–64.
Karimi M, Ghasemi A, Zangabad PS, Rahighi R, Basri SMM, Mirshekari H, et al. Smart micro/nanoparticles in stimulus-responsive drug/gene delivery systems. Chem Soc Rev. 2016;45(5):1457–501.
Google Scholar
Chavanpatil MD, Khdair A, Panyam J. Nanoparticles for cellular drug delivery: mechanisms and factors influencing delivery. J Nanosci Nanotechnol. 2006;6(9–10):2651–63.
Google Scholar
Anarjan FS. Active targeting drug delivery nanocarriers: Ligands. Nano Struct Nano Objects. 2019;19:100370.
Pack DW, Hoffman AS, Pun S, Stayton PS. Design and development of polymers for gene delivery. Nat Rev Drug Discovery. 2005;4(7):581–93.
Google Scholar
Almeciga-Diaz CJ, Barrera LA. Design and applications of gene therapy vectors for mucopolysaccharidosis in Colombia. Gene Ther. 2020;27(1):104–7.
Google Scholar
Leal AF, Cifuentes J, Torres CE, Suárez D, Quezada V, Gómez SC, et al. Delivery and assessment of a CRISPR/nCas9-based genome editing system on in vitro models of mucopolysaccharidoses IVA assisted by magnetite-based nanoparticles. Sci Rep. 2022;12(1):15045.
Google Scholar
Zhang Q, Kuang G, Li W, Wang J, Ren H, Zhao Y. Stimuli-responsive gene delivery nanocarriers for cancer therapy. Nano Micro Lett. 2023;15(1):44.
Brooks BD, Brooks AE. Therapeutic strategies to combat antibiotic resistance. Adv Drug Deliv Rev. 2014;78:14–27.
Google Scholar
Slavin YN, Asnis J, Hńfeli UO, Bach H. Metal nanoparticles: understanding the mechanisms behind antibacterial activity. J Nanobiotechnology. 2017;15:1–20.
Nagarajan P, Subramaniyan V, Elavarasan V, Mohandoss N, Subramaniyan P, Vijayakumar S. Biofabricated aluminium oxide nanoparticles derived from Citrus aurantium L.: antimicrobial, anti-proliferation, and photocatalytic efficiencies. Sustainability. 2023;15(2):1743.
Google Scholar
Flores-López LZ, Espinoza-Gómez H, Somanathan R. Silver nanoparticles: electron transfer, reactive oxygen species, oxidative stress, beneficial and toxicological effects. J Appl Toxicol. 2019;39(1):16–26.
Google Scholar
Khan SS, Ullah I, Ullah S, An R, Xu H, Nie K, et al. Recent advances in the surface functionalization of nanomaterials for antimicrobial applications. Materials. 2021;14(22):6932.
Google Scholar
Fabrega J, Fawcett SR, Renshaw JC, Lead JR. Silver nanoparticle impact on bacterial growth: effect of pH, concentration, and organic matter. Environ Sci Technol. 2009;43(19):7285–90.
Google Scholar
Murthy SK. Nanoparticles in modern medicine: state of the art and future challenges. Int J Nanomed. 2007;2(2):129–41.
Google Scholar
Chen Z, Liu F, Chen Y, Liu J, Wang X, Chen AT, et al. Targeted delivery of CRISPR/Cas9-mediated cancer gene therapy via liposome-templated hydrogel nanoparticles. Adv Funct Mater. 2017;27(46):1703036.
Google Scholar
Chowdhry R, Lu SZ, Lee S, Godhulayyagari S, Ebrahimi SB, Samanta D. Enhancing CRISPR/Cas systems with nanotechnology. Trends Biotechnol. 2023;41(12):1549–64.
Google Scholar
Saw PE, Cui Gh, Xu X. Nanoparticles-mediated CRISPR/Cas gene editing delivery system. ChemMedChem. 2022;17(9):e202100777.
Google Scholar
Pandey P, Vavilala SL. From gene editing to biofilm busting: CRISPR-CAS9 against antibiotic resistance—a review. Cell Biochem Biophys. 2024;82(2):549–60.
Google Scholar
Albanese A, Tang PS, Chan WCW. The effect of nanoparticle size, shape, and surface chemistry on biological systems. Annu Rev Biomed Eng. 2012;14:1–16.
Google Scholar
Blanco E, Shen H, Ferrari M. Principles of nanoparticle design for overcoming biological barriers to drug delivery. Nat Biotechnol. 2015;33(9):941–51.
Google Scholar
Schrand AM, Rahman MF, Hussain SM, Schlager JJ, Smith DA, Syed AF. Metal-based nanoparticles and their toxicity assessment. WIREs Nanomed Nanobiotechnol. 2010;2(5):544–68.
Google Scholar
Fujii E, Kimura K, Mizoguchi K, Kato A, Takanashi H, Itoh Z, et al. Assessment of the carcinogenic potential of mitemcinal (GM-611): increased incidence of malignant lymphoma in a rat carcinogenicity study. Toxicol Appl Pharmacol. 2008;228(1):1–7.
Google Scholar
Kara G, Calin GA, Ozpolat B. Rnai-based therapeutics and tumor targeted delivery in cancer. Adv Drug Deliv Rev. 2022;182:114113.
Google Scholar
Tu K, Deng H, Kong L, Wang Y, Yang T, Hu Q, et al. Reshaping tumor immune microenvironment through acidity-responsive nanoparticles featured with CRISPR/Cas9-mediated programmed death-ligand 1 attenuation and chemotherapeutics-induced immunogenic cell death. ACS Appl Mater Interfaces. 2020;12(14):16018–30.
Google Scholar
Xie R, Wang Y, Gong S. External stimuli-responsive nanoparticles for spatially and temporally controlled delivery of CRISPR-Cas genome editors. Biomater Sci. 2021;9(18):6012–22.
Google Scholar
Zhao Z, Ding C, Wang Y, Tan H, Li J. PH-Responsive polymeric nanocarriers for efficient killing of cariogenic bacteria in biofilms. Biomater Sci. 2019;7:1643–51.
Google Scholar
Cheng MHY, Leung J, Zhang Y, Strong C, Basha G, Momeni A, et al. Induction of bleb structures in lipid nanoparticle formulations of mRNA leads to improved transfection potency. Adv Mater. 2023;35(31):2303370.
Google Scholar
Chan Y-T, Lu Y, Wu J, Zhang C, Tan H-Y, Bian Z-x, et al. CRISPR-Cas9 library screening approach for anti-cancer drug discovery: overview and perspectives. Theranostics. 2022;12(7):3329.
Google Scholar
Ding X, Yin B, Qian L, Zeng Z, Yang Z, Li H, et al. Screening for novel quorum-sensing inhibitors to interfere with the formation of Pseudomonas aeruginosa biofilm. J Med Microbiol. 2011;60(12):1827–34.
Google Scholar
De Kievit T. Quorum sensing in Pseudomonas aeruginosa biofilms. Environ Microbiol. 2009;11(2):279–88.
Google Scholar
Ates A, Tastan C, Ermertcan S. CRISPR-Cas9-mediated targeting of multidrug resistance genes in methicillin-resistant Staphylococcus aureus. CRISPR J. 2024;7(6):374–84.
Google Scholar
Agha ASA, Al-Samydai A, Aburjai T. New frontiers in CRISPR: Addressing antimicrobial resistance with Cas9, Cas12, Cas13, and Cas14. Heliyon. 2025;11:e42013.
Knott GJ, Doudna JA. CRISPR-Cas guides the future of genetic engineering. Science. 2018;361(6405):866–9.
Google Scholar
Gholizadeh P, Aghazadeh M, Ghotaslou R, Rezaee MA, Pirzadeh T, Cui L, et al. Role of CRISPR-Cas system on antibiotic resistance patterns of Enterococcus faecalis. Ann Clin Microbiol Antimicrob. 2021;20:1–12.
Abdul R, Wang M-R, Zhong C-J, Liu Y-Y, Hou W, Xiong H-R. An updated review on the antimicrobial and pharmacological properties of Uncaria (Rubiaceae). J Herbal Med. 2022;34:100573.
Zhang F, Wen Y, Guo X. CRISPR/Cas9 for genome editing: progress, implications and challenges. Hum Mol Genet. 2014;23(R1):R40–6.
Google Scholar
Wang S-W, Gao C, Zheng Y-M, Yi L, Lu J-C, Huang X-Y, et al. Current applications and future perspective of CRISPR/Cas9 gene editing in cancer. Mol Cancer. 2022;21(1):57.
Google Scholar
Savić N, Schwank G. Advances in therapeutic CRISPR/Cas9 genome editing. Transl Res. 2016;168:15–21.
Google Scholar
Makarova KS, Wolf YI, Alkhnbashi OS, Costa F, Shah SA, Saunders SJ, et al. An updated evolutionary classification of CRISPR-Cas systems. Nat Rev Microbiol. 2015;13(11):722–36.
Google Scholar
Makarova KS, Wolf YI, Iranzo J, Shmakov SA, Alkhnbashi OS, Brouns SJJ, et al. Evolutionary classification of CRISPR-Cas systems: a burst of class 2 and derived variants. Nat Rev Microbiol. 2020;18(2):67–83.
Google Scholar
Morisaka H, Yoshimi K, Okuzaki Y, Gee P, Kunihiro Y, Sonpho E, et al. Crispr-Cas3 induces broad and unidirectional genome editing in human cells. Nat Commun. 2019;10(1):5302.
Google Scholar
Rostøl JT, Xie W, Kuryavyi V, Maguin P, Kao K, Froom R, et al. The Card1 nuclease provides defence during type III CRISPR immunity. Nature. 2021;590(7847):624–9.
Google Scholar
Lin J, Fuglsang A, Kjeldsen AL, Sun K, Bhoobalan-Chitty Y, Peng X. DNA targeting by subtype I-D CRISPR-Cas shows type I and type III features. Nucleic Acids Res. 2020;48(18):10470–8.
Google Scholar
Jackson RN, Golden SM, van Erp PB, Carter J, Westra ER, Brouns SJ, et al. Structural biology. Crystal structure of the CRISPR RNA-guided surveillance complex from Escherichia coli. Science. 2014;345(6203):1473–9.
Google Scholar
Karneyeva K, Kolesnik M, Livenskyi A, Zgoda V, Zubarev V, Trofimova A, et al. Interference requirements of type III CRISPR-Cas systems from Thermus thermophilus. J Mol Biol. 2024;436(6):168448.
Google Scholar
Rostøl JT, Marraffini LA. Non-specific degradation of transcripts promotes plasmid clearance during type III-A CRISPR-Cas immunity. Nat Microbiol. 2019;4(4):656–62.
Google Scholar
Koonin EV, Makarova KS. Origins and evolution of CRISPR-Cas systems. Philos Trans R Soc Lond B Biol Sci. 2019;374(1772):20180087.
Google Scholar
Moya-Beltrán A, Makarova KS, Acuña LG, Wolf YI, Covarrubias PC, Shmakov SA, et al. Evolution of type IV CRISPR-Cas systems: insights from CRISPR loci in integrative conjugative elements of Acidithiobacillia. Crispr j. 2021;4(5):656–72.
Google Scholar
Pinilla-Redondo R, Mayo-Muñoz D, Russel J, Garrett RA, Randau L, Sørensen SJ, et al. Type IV CRISPR-Cas systems are highly diverse and involved in competition between plasmids. Nucleic Acids Res. 2020;48(4):2000–12.
Google Scholar
Faure G, Makarova KS, Koonin EV. CRISPR-Cas: complex functional networks and multiple roles beyond adaptive immunity. J Mol Biol. 2019;431(1):3–20.
Google Scholar
Jiang F, Doudna JA. CRISPR-Cas9 structures and mechanisms. Annu Rev Biophys. 2017;46:505–29.
Google Scholar
Shmakov S, Smargon A, Scott D, Cox D, Pyzocha N, Yan W, et al. Diversity and evolution of class 2 CRISPR-Cas systems. Nat Rev Microbiol. 2017;15(3):169–82.
Google Scholar
Yosef I, Goren MG, Qimron U. Proteins and DNA elements essential for the CRISPR adaptation process in Escherichia coli. Nucleic Acids Res. 2012;40(12):5569–76.
Google Scholar
Bikard D, Euler CW, Jiang W, Nussenzweig PM, Goldberg GW, Duportet X, et al. Exploiting CRISPR-Cas nucleases to produce sequence-specific antimicrobials. Nat Biotechnol. 2014;32(11):1146–50.
Google Scholar
Doudna JA, Charpentier E. Genome editing. The new frontier of genome engineering with CRISPR-Cas9. Science. 2014;346(6213):1258096.
Google Scholar
Ledford H. Major CRISPR patent decision won’t end tangled dispute. Nature. 2022;603(7901):373–4.
Google Scholar
Koonin EV, Makarova KS, Zhang F. Diversity, classification and evolution of CRISPR-Cas systems. Curr Opin Microbiol. 2017;37:67–78.
Google Scholar
Yan WX, Hunnewell P, Alfonse LE, Carte JM, Keston-Smith E, Sothiselvam S, et al. Functionally diverse type V CRISPR-Cas systems. Science. 2019;363(6422):88–91.
Google Scholar
Chen JS, Ma E, Harrington LB, Da Costa M, Tian X, Palefsky JM, et al. CRISPR-Cas12a target binding unleashes indiscriminate single-stranded DNase activity. Science. 2018;360(6387):436–9.
Google Scholar
Li SY, Cheng QX, Wang JM, Li XY, Zhang ZL, Gao S, et al. CRISPR-Cas12a-assisted nucleic acid detection. Cell Discov. 2018;4:20.
Google Scholar
Li L, Li S, Wu N, Wu J, Wang G, Zhao G, et al. HOLMESv2: a CRISPR-Cas12b-assisted platform for nucleic acid detection and DNA methylation quantitation. ACS Synth Biol. 2019;8(10):2228–37.
Google Scholar
Broughton JP, Deng X, Yu G, Fasching CL, Servellita V, Singh J, et al. CRISPR-Cas12-based detection of SARS-CoV-2. Nat Biotechnol. 2020;38(7):870–4.
Google Scholar
Rananaware SR, Meister KS, Shoemaker GM, Vesco EK, Sandoval LSW, Lewis JG, et al. PAM-free diagnostics with diverse type V CRISPR-Cas systems. medRxiv. 2024.
Kordyś M, Sen R, Warkocki Z. Applications of the versatile CRISPR-Cas13 RNA targeting system. WIREs RNA. 2022;13(3):e1694.
Google Scholar
O’Connell MR. Molecular mechanisms of RNA targeting by Cas13-containing type VI CRISPR-Cas systems. J Mol Biol. 2019;431(1):66–87.
Google Scholar
Abudayyeh OO, Gootenberg JS, Konermann S, Joung J, Slaymaker IM, Cox DB, et al. C2c2 is a single-component programmable RNA-guided RNA-targeting CRISPR effector. Science. 2016;353(6299):aaf5573.
Google Scholar
Gootenberg JS, Abudayyeh OO, Kellner MJ, Joung J, Collins JJ, Zhang F. Multiplexed and portable nucleic acid detection platform with Cas13, Cas12a, and Csm6. Science. 2018;360(6387):439–44.
Google Scholar
Abudayyeh OO, Gootenberg JS, Essletzbichler P, Han S, Joung J, Belanto JJ, et al. RNA targeting with CRISPR-Cas13. Nature. 2017;550(7675):280–4.
Google Scholar
Kellner MJ, Koob JG, Gootenberg JS, Abudayyeh OO, Zhang F. SHERLOCK: nucleic acid detection with CRISPR nucleases. Nat Protoc. 2019;14(10):2986–3012.
Google Scholar
Altae-Tran H, Kannan S, Suberski AJ, Mears KS, Demircioglu FE, Moeller L, et al. Uncovering the functional diversity of rare CRISPR-Cas systems with deep terascale clustering. Science. 2023;382(6673):eadi1910.
Google Scholar
Smargon AA, Cox DBT, Pyzocha NK, Zheng K, Slaymaker IM, Gootenberg JS, et al. Cas13b Is a Type VI-B CRISPR-Associated RNA-Guided RNase Differentially Regulated by Accessory Proteins Csx27 and Csx28. Mol Cell. 2017;65(4):618-30.e7.
Google Scholar
Hong T, Luo Q. Advances in the RNA-targeting CRISPR-Cas systems. Sheng Wu Gong Cheng Xue Bao. 2023;39(4):1363–73.
Google Scholar
Perčulija V, Lin J, Zhang B, Ouyang S. Functional features and current applications of the RNA-targeting type VI CRISPR-Cas systems. Adv Sci. 2021;8(13):2004685.
Yan WX, Chong S, Zhang H, Makarova KS, Koonin EV, Cheng DR, et al. Cas13d is a compact RNA-targeting type VI CRISPR effector positively modulated by a WYL-domain-containing accessory protein. Mol Cell. 2018;70(2):327-39.e5.
Google Scholar
Pinto-Alphandary H, Andremont A, Couvreur P. Targeted delivery of antibiotics using liposomes and nanoparticles: research and applications. Int J Antimicrob Agents. 2000;13(3):155–68.
Google Scholar
Tyagi P, Wu P-C, Chancellor M, Yoshimura N, Huang L. Recent advances in intravesical drug/gene delivery. Mol Pharm. 2006;3(4):369–79.
Google Scholar
Yang Y, Wang D, Lü P, Ma S, Chen K. Research progress on nucleic acid detection and genome editing of CRISPR/Cas12 system. Mol Biol Rep. 2023;50(4):3723–38.
Google Scholar
Doudna JA, Charpentier E. The new frontier of genome engineering with CRISPR-Cas9. Science. 2014;346(6213):1258096.
Google Scholar