UCL and Great Ormond Street Hospital have developed the world’s first gene therapy for p47 Chronic Granulomatous Disease, providing life-changing treatment for patients with this rare immune disorder
Researchers at University College London have developed a groundbreaking gene therapy that offers new hope for patients living with p47 Chronic Granulomatous Disease. This innovative approach represents a significant advancement in the treatment of life-limiting diseases and could pave the way for future therapies that transform patient outcomes.
p47 Chronic Granulomatous Disease: A rare immune condition affecting one in a million
p47 Chronic Granulomatous Disease is an inherited, rare genetic immunodeficiency disorder. People with this condition have immune systems that do not function properly, leaving them susceptible to inflammation and frequent bacterial and fungal infections. This condition can leave patients with severe life-limiting conditions such as colitis, inflammatory bowel disease, and inflammatory complications, and in some cases, can be life-threatening due to the risk of severe infections.
p47 Chronic Granulomatous Disease is currently treated with a bone marrow transplant; however, finding a matching donor is a challenging and long-winded experience. Previous gene therapy research has proven successful for a different form of Chronic Granulomatous Disease called X-CGD, but there have been no trials for the p47 form.
Gene therapy developed and delivered under one roof
The p47 gene therapy was also the first product to be researched, manufactured, and delivered to patients under one roof at the Zayed Centre for Research into Rare Disease in Children, run jointly by UCL and GOSH.
To deliver the gene therapy to a patient, a viral vector is required, which is a harmless virus that ‘tricks’ cells into accepting new genes. These are highly complex to make, and their availability and effectiveness dictate whether a clinical trial of gene or cell therapy will work.
In 2023, GOSH received an MHRA licence to manufacture viral vectors in the Zayed Centre, and the UCL Technology Fund, managed by Albion VC in collaboration with UCL Business, funded the development of the vector, the preclinical testing, and the ongoing clinical trial.
The gene therapy went through several quality assurance checks and was then handed over to the clinical trial delivery team responsible for administering the treatment to patients in the trial.
The trial’s Principal Investigator, Professor Claire Booth, Mahboubian Professor in Gene Therapy at UCL Great Ormond Institute of Child Health and Consultant in Paediatric Immunology at GOSH, who led the clinical trial said: “For the first time we’ve been able to develop, manufacture, and deliver a new gene therapy entirely under one roof – marking a true bench-to-bedside milestone.
“This is a significant step forward. It means that future gene therapies developed at UCL and GOSH could reach clinical trials, and patients with rare diseases like Remi, faster than ever before.”
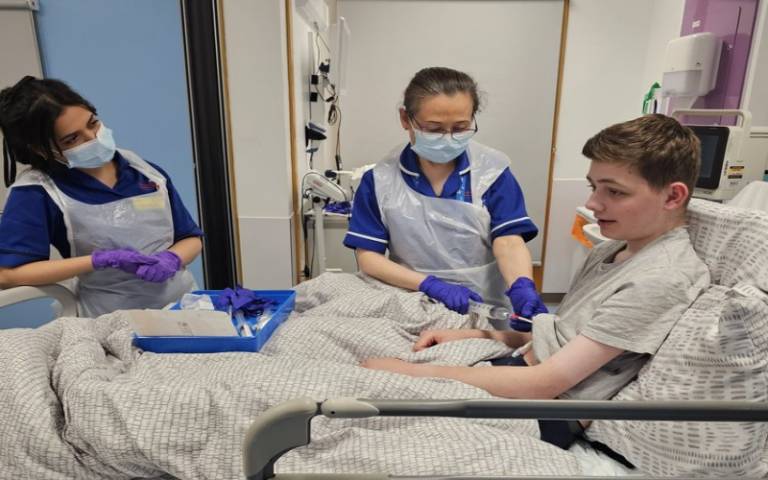
World’s first gene therapy gives 19-year-old patient a new lease on life
Remi, aged 19, is the first patient to receive this gene therapy in the world, which was developed by a team at UCL Great Ormond Street Institute of Child Health, led by Professor Adrian Thrasher and Dr Georgia Santilli.
Remi was first diagnosed with Chronic Granulomatous Disease in 2007 and had been waiting for a bone marrow transplant match when he found out about the trial. “Before the trial, I became unwell and was struggling with my weight due to inflammation in my gut. I had been missing a lot of school, and even when doing online studies, it was hard to focus when I was in the hospital,“ he said. “Having the gene therapy has completely changed my life. I can go out and about now without worrying, help my family out, and I’m excited to start university and start the next stage of my life.”