Locomotor activity(open field)
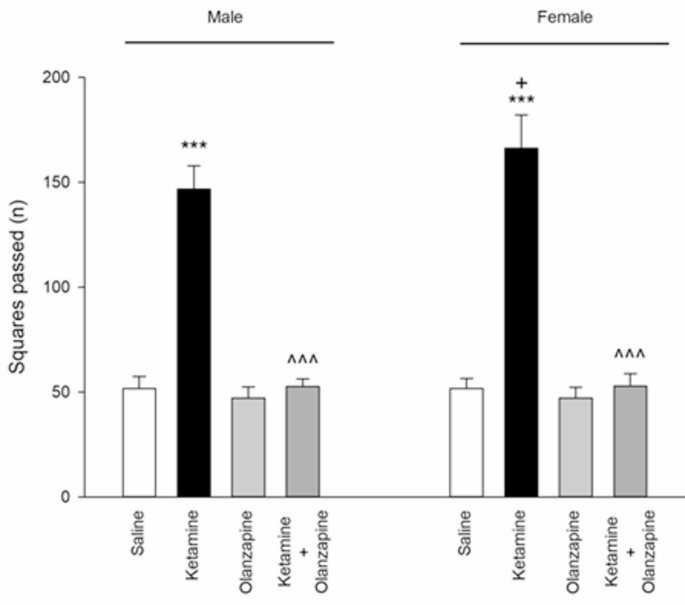
Ketamine treatment significantly affected locomotor activity in both male and female rats (one-way ANOVA within each sex: F3, 24 = 336.6 for males, F3, 24 = 277.6 for females; p < 0.001 for both). Post-hoc tests showed that ketamine (30 mg/kg) caused a dramatic increase in locomotor activity compared to saline controls in both males and females (**p < 0.001 vs. saline; Fig. 1). **Olanzapine treatment effectively reduced locomotor activity in ketamine-treated rats, bringing it back down toward control levels in both sexes (p < 0.001 for ket + olanzapine vs. ketamine alone). Importantly, olanzapine alone (without ketamine) did not significantly differ from saline in locomotor counts for either sex (p > 0.05 vs. saline), indicating that olanzapine by itself did not suppress normal locomotion.
Squares passed (locomotor activity) in both males and females received saline, ketamine, olanzapine, and both (***P < 0.001 compared with related saline; ^^^P < 0.001 compared with related ketamine; +P < 0.05 compared with respective male group; n = 7).
When considering sex as a factor (two-way ANOVA: Treatment × Sex), there was a significant interaction (F3, 48 = 4.96, p < 0.01) and a main effect of sex (F1, 48 = 5.05, p < 0.05) on locomotor activity. Female rats exhibited higher locomotor activity than males under ketamine treatment (p < 0.05 for female-ketamine vs. male-ketamine; Fig. 1), even though both were elevated relative to controls. No sex difference was observed in saline or olanzapine-only groups. These results indicate that ketamine induced hyperlocomotion in both sexes, with females showing a greater hyperactive response, and that olanzapine reversed ketamine-induced hyperlocomotion irrespective of sex.
Rearing behavior(open field)
Ketamine also significantly influenced rearing (exploratory behavior) in the open field (one-way ANOVA: F3, 24 = 78.7 for males, F3, 24 = 322.6 for females; p < 0.001). Ketamine markedly decreased the number of rears compared to saline in both males and females (**p < 0.001 vs. saline; Fig. 2), suggesting increased anxiety-like behavior or decreased exploration. Olanzapine plus ketamine partially attenuated this effect, but in a sex-dependent manner. In female rats, ketamine + olanzapine showed significantly more rearing activity than ketamine alone (+++**p < 0.001 vs. ketamine), indicating that olanzapine restored exploratory behavior in females nearly to control levels (although still slightly lower than saline females, ++**p < 0.001 vs. saline, implying a partial restoration). In male rats, however, olanzapine did not significantly increase rearing compared to ketamine alone (p > 0.05), indicating no notable improvement of rearing in males. Olanzapine-only groups did not differ from saline in rearing for either sex.
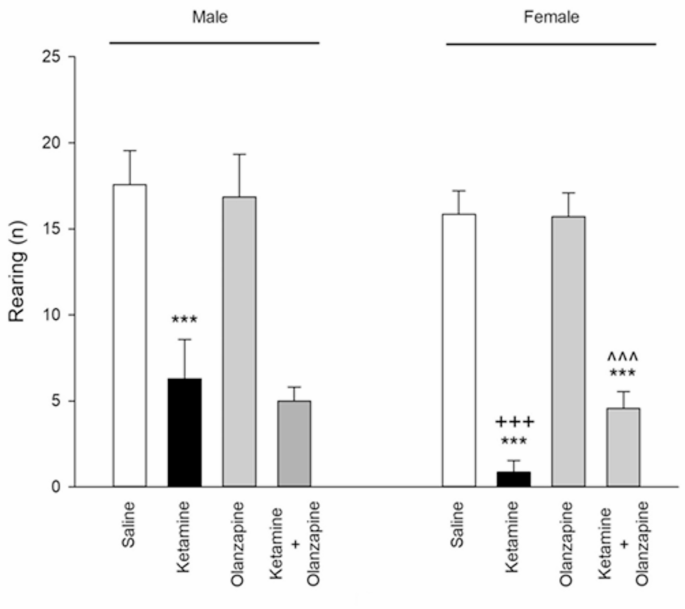
Rearing behavior (anxiety-like behavior) in both males and females received saline, ketamine, olanzapine, and both (***P < 0.001 compared with related saline; ^^^P < 0.001 compared with related ketamine; +++P < 0.001 compared with respective male group; n = 7).
Two-way ANOVA showed a significant Treatment × Sex interaction for rearing (F3, 48 = 6.58, p < 0.001) as well as main effects of Treatment (F3, 48 = 269.5, p < 0.001) and Sex (F1, 48 = 25.14, p < 0.001). Ketamine’s effect on rearing was significantly more pronounced in females than in males, as evidenced by female ketamine rats rearing less than male ketamine rats (+++**p < 0.001 female vs. male ketamine; Fig. 2). This aligns with the locomotor findings: female rats appear more affected by ketamine in open-field behaviors (greater hyperactivity and greater reduction in exploration). Olanzapine’s efficacy in alleviating the rearing deficit was observed only in females, highlighting a sex-specific therapeutic effect on this anxiety-related measure.
Pain threshold
Ketamine had a significant effect on pain sensitivity, but only in females. Among female rats, there were significant differences between treatment groups (F3, 24 = 63.49, p < 0.001), whereas in males the group differences were not significant (F3, 24 = 0.91, p > 0.05; one-way ANOVAs). Female rats treated with ketamine showed a marked decrease in hot plate latency compared to saline controls (**p < 0.001; Fig. 3), indicating hyperalgesia (lower pain threshold). In contrast, male rats did not show a significant change in latency with ketamine (their latency was similar to saline controls, p > 0.05). Olanzapine co-treatment completely reversed the ketamine-induced hyperalgesia in females: ketamine + olanzapine females had significantly higher latencies than ketamine-alone females (^^^**p < 0.001 vs. ketamine) and were statistically indistinguishable from saline females (p > 0.05 vs. saline), indicating restoration of normal pain threshold. Olanzapine alone had no effect on pain threshold in either sex (no difference vs. saline).
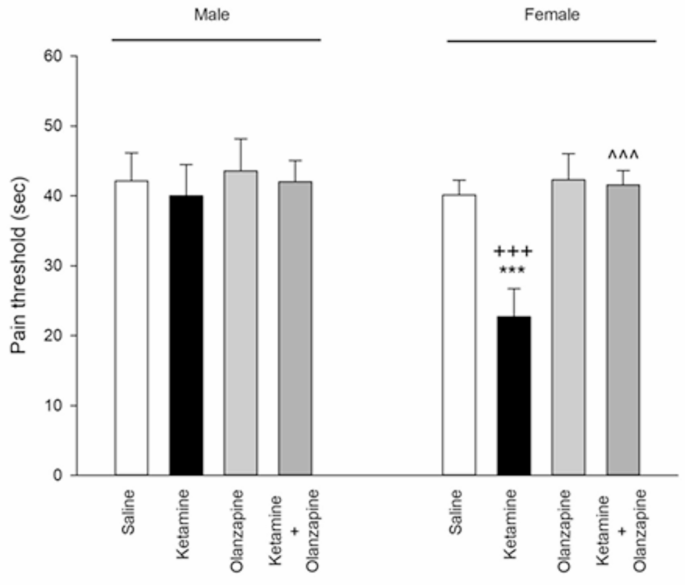
Pain threshold in both males and females received saline, ketamine, olanzapine, and both (***P < 0.001 compared with related saline; ^^^P < 0.001 compared with related ketamine; +++P < 0.001 compared with respective male group; n = 7).
Two-way ANOVA (Treatment × Sex) for hot plate latency revealed a significant main effect of Treatment (F3, 48 = 30.52, p < 0.001) and Sex (F1, 48 = 29.40, p < 0.001), as well as a significant interaction (F3, 48 = 17.28, p < 0.001). Post-hoc analysis confirmed that ketamine’s effect was significant in females but not in males, yielding a significant difference between ketamine females vs. ketamine males (+++**p < 0.001; Fig. 3). These results demonstrate a female-specific hyperalgesic effect of ketamine, which was mitigated by olanzapine. In males, pain perception was unaffected by ketamine in this paradigm, hence no rescue was needed. This sex difference in nociceptive response to ketamine is a novel finding in our study.
OCD-like behavior(Marble Burying)
Ketamine did not significantly affect marble burying behavior in either sex. The number of marbles buried showed no significant differences between any of the groups for males (F3, 24 = 0.17, p > 0.05) or females (F3, 24 = 0.12, p > 0.05) in one-way ANOVAs. Across treatments, rats buried a comparable number of marbles (on average, 2–4 marbles out of 10) regardless of ketamine or olanzapine. Two-way ANOVA similarly indicated no significant main effects or interaction (Treatment: F3, 48 = 0.09; Sex: F1, 48 = 0.01; interaction: F3, 48 = 0.19; all p > 0.05). There was no observable sex difference and no impact of ketamine or olanzapine on this compulsive-like digging measure (Fig. 4). These results suggest that sub-chronic ketamine at this dose did not induce an increase in repetitive digging or anxiety-driven burying behavior, and olanzapine had no effect either. The lack of change in marble burying aligns with the idea that not all behaviors are affected by the ketamine model, or it might indicate that any potential increase in compulsivity by ketamine was offset by general hyperactivity changes. In summary, no OCD-like phenotype was detected under our experimental conditions.
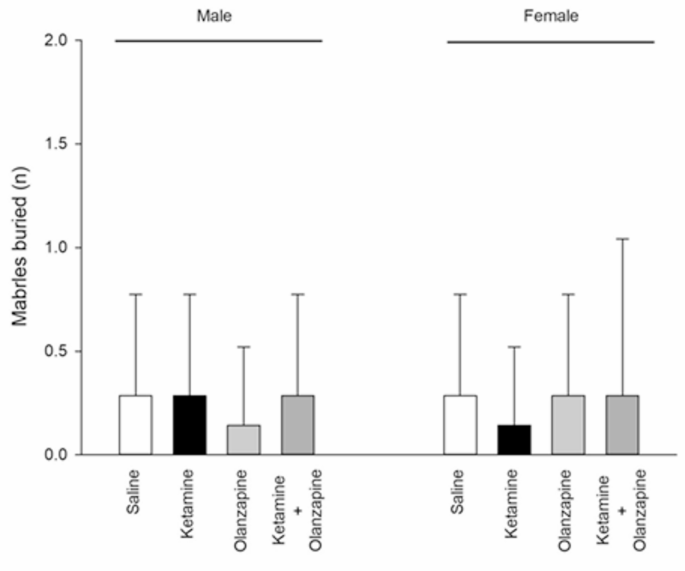
Marbles buried (OCD-like behavior) in both males and females received saline, ketamine, olanzapine, and both (n = 7).
Immobility (forced swim test)
Ketamine’s effects on depressive-like behavior were sex-specific. In female rats, ketamine caused a clear reduction in immobility time in the FST compared to saline (**p < 0.001; Fig. 5), which can be interpreted as an antidepressant-like effect or increased active coping (consistent with ketamine’s known rapid antidepressant action). Female ketamine rats spent more time struggling (climbing/swimming) and less time immobile than control females. In male rats, ketamine did not significantly change immobility time (p > 0.05 vs. saline). Consequently, a one-way ANOVA showed significant group differences in females (F3, 24 = 98.11, p < 0.001) but not in males (F3, 24 = 2.16, p = 0.12). Olanzapine co-treatment in females increased immobility time back to near control levels: ketamine + olanzapine females had significantly higher immobility than ketamine-alone females (^^^**p < 0.001 vs. ketamine), indicating that olanzapine reversed ketamine’s antidepressant-like effect in females (i.e., it normalized the behavior, as ketamine-alone females were unusually low in immobility). Ketamine + olanzapine female immobility did not differ from saline female immobility (p > 0.05), showing full restoration. In males, olanzapine + ketamine produced no notable change since ketamine alone had no effect (male ketamine vs. male ket + olz, p > 0.05). Olanzapine alone did not significantly affect immobility in either sex (no difference vs. saline).
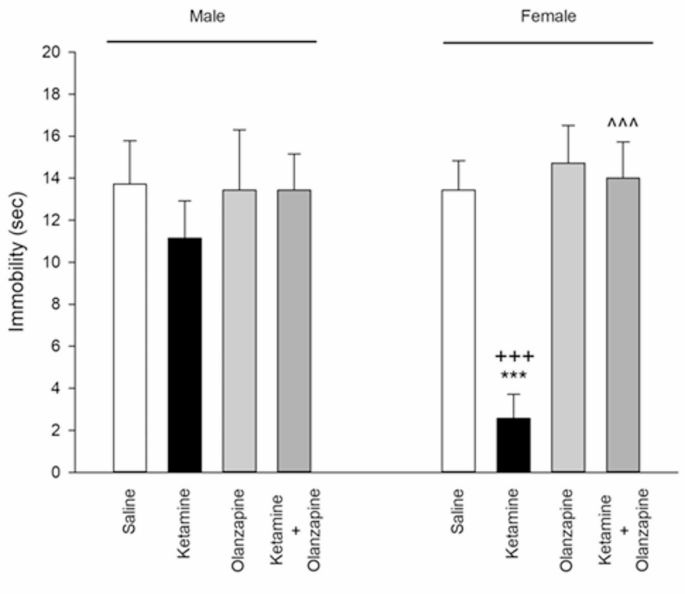
Immobility (depressive-like behavior) in both males and females received saline, ketamine, olanzapine, and both (***P < 0.001 compared with related saline; ^^^P < 0.001 compared with related ketamine; +++P < 0.001 compared with respective male group; n = 7).
Two-way ANOVA (Treatment × Sex) for FST immobility yielded a significant Treatment effect (F3, 48 = 48.02, p < 0.001), Sex effect (F1, 48 = 12.21, p < 0.001), and interaction (F3, 48 = 21.02, p < 0.001). Ketamine-treated females had substantially lower immobility than ketamine-treated males (+++**p < 0.001 female vs. male ketamine; Fig. 5). This reinforces that ketamine’s impact in the FST was observed in females only. The fact that ketamine decreased immobility in females suggests a peculiar antidepressant-like action in female rats, which might be related to their increased ketamine sensitivity or hormonal status (explored in Discussion). Olanzapine by itself did not alter FST behavior, but in combination with ketamine, it nullified ketamine’s effect in females, leading to no sex difference in the ket + olanzapine group.
Climbing (forced swim test)
Ketamine increased climbing behavior (active escape efforts) in the FST in both sexes. One-way ANOVA showed significant treatment effects for both males (F3, 24 = 11.99, p < 0.001) and females (F3, 24 = 14.40, p < 0.001). Post-hoc comparisons indicated that ketamine-treated rats spent more time actively climbing compared to saline controls in both males and females (**p < 0.001 vs. saline; Fig. 6). This is consistent with the reduced immobility (especially in females), as climbing is an alternative behavior in the FST. Olanzapine co-administration prevented the ketamine-induced increase in climbing: in both sexes, the ketamine + olanzapine groups had significantly lower climbing times than the ketamine-alone groups (^^^**p < 0.001 vs. ketamine). In fact, with olanzapine, climbing behavior returned to control-like levels (no significant difference between ket + olanzapine and saline in either sex, p > 0.05). Olanzapine-only rats showed climbing times similar to saline.
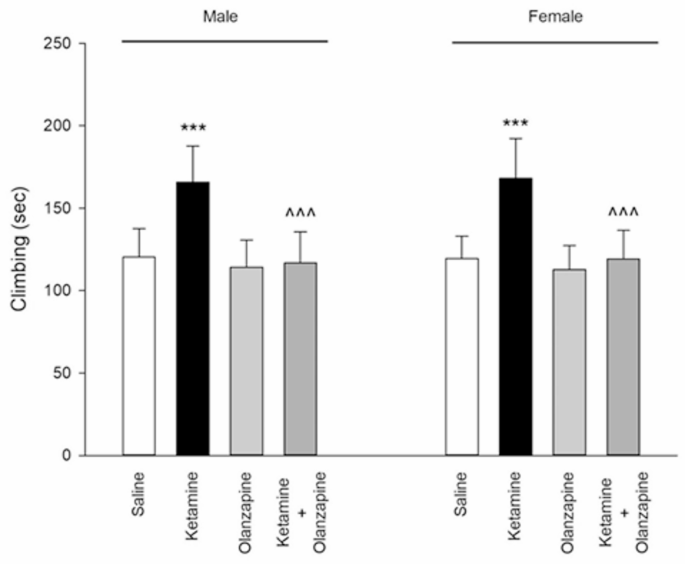
Climbing in both males and females received saline, ketamine, olanzapine, and both (***P < 0.001 compared with related saline; ^^^P < 0.001 compared with related ketamine; n = 7).
Unlike immobility, no sex difference was detected in climbing. Two-way ANOVA revealed a significant main effect of Treatment (F3, 48 = 26.24, p < 0.001) on climbing, but the effect of Sex was not significant (F1, 48 = 0.02, p = 0.88) and neither was the interaction (F3, 48 = 0.04, p = 0.99). Thus, ketamine increased climbing to a similar extent in males and females, and olanzapine reduced it similarly in both (Fig. 6). This indicates that for this particular active coping behavior, ketamine’s impact was robust and sex-independent, and olanzapine’s efficacy in countering that impact was also equal in males and females. In summary, ketamine stimulated more escape-oriented behavior in the FST, and olanzapine co-treatment normalized this behavior.
Novel object recognition memory
Ketamine had a profound effect on recognition memory, as indicated by the Discrimination Index (DI) in the novel object recognition test. One-way ANOVA within each sex showed significant group effects (F3, 24 = 177.7, p < 0.001 for males; F3, 24 = 140.2, p < 0.001 for females). Saline-treated rats of both sexes showed a strong preference for the novel object (positive DI, indicating normal recognition memory). In contrast, ketamine-treated rats had a significantly lower DI than controls (**p < 0.001 vs. saline), indicating impaired recognition memory (Fig. 7). In fact, ketamine rats spent roughly equal time on novel and familiar objects, resulting in DIs near zero or slightly negative (some ketamine rats explored the familiar object as much or more than the novel, suggesting memory deficit or neophobia). Olanzapine improved recognition memory in ketamine-treated rats of both sexes: the ketamine + olanzapine groups showed higher DIs than the ketamine-alone groups (^^^**p < 0.001 vs. ketamine). This suggests that olanzapine partially attenuated the memory impairment. However, ketamine + olanzapine DIs were still somewhat lower than saline in both sexes (+ + p < 0.01 vs. saline in post-hoc), indicating memory was not fully restored to normal levels, but the deficit was significantly reduced. Olanzapine alone did not significantly affect DI compared to saline (p > 0.05), implying it does not alter memory in untreated rats.
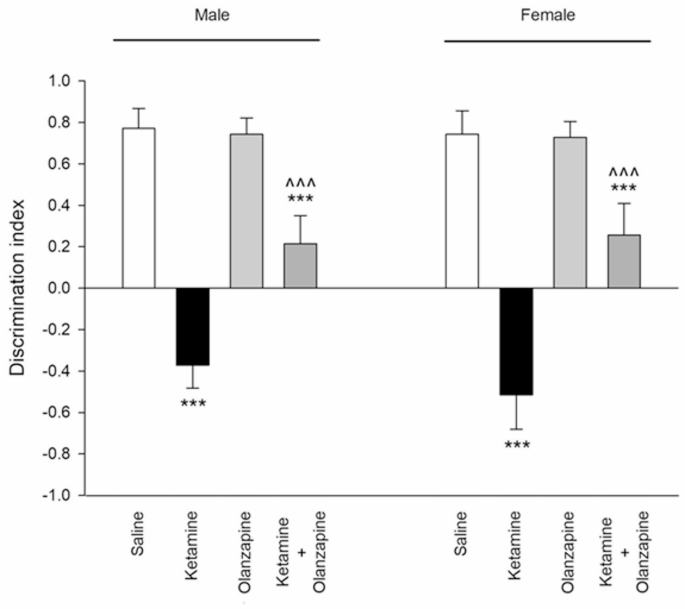
Discrimination index (novel object recognition memory) in both males and females received saline, ketamine, olanzapine, and both (***P < 0.001 compared with related saline; ^^^P < 0.001 compared with related ketamine; n = 7).
Two-way ANOVA (Treatment × Sex) for the discrimination index showed a strong Treatment effect (F3, 48 = 308.7, p < 0.001). The Sex effect was not significant (F1, 48 = 1.24, p = 0.27) and the interaction was not significant (F3, 48 = 1.47, p = 0.23). This indicates that male and female rats were similarly affected by ketamine and olanzapine in the NORT (Fig. 7). Both sexes had impaired memory with ketamine and both benefitted from olanzapine to a similar degree. There was no detectable sex difference in novel object recognition performance under any condition in our data. Thus, ketamine induced a severe recognition memory deficit in both sexes, and olanzapine partially reversed this deficit in both sexes. The persistence of some impairment in the ket + olanzapine group may suggest that the 6 mg/kg olanzapine only partially counteracts the cognitive effects of this ketamine regimen.
BDNF mRNA expression (prefrontal cortex)
In the saline control groups, there were no sex differences in baseline BDNF mRNA levels in the PFC (by design we normalized each sex to its control). Ketamine administration significantly decreased BDNF mRNA expression in the prefrontal cortex of both male and female rats (Fig. 8). One-way ANOVA on BDNF levels (expressed as % of same-sex control) showed a significant effect of treatment in males (F2, 6 = 7.07, p < 0.05) and in females (F2,6 = 6.96, p < 0.05). Post-hoc tests confirmed that ketamine-only groups had significantly lower BDNF expression relative to saline controls (p < 0.05 in males, p < 0.01 in females). BDNF was about 60–70% of control levels in ketamine-treated rats, consistent with previous findings of ketamine’s downregulation of neurotrophic factors6,40. Importantly, olanzapine co-treatment did not significantly increase BDNF mRNA compared to ketamine alone in either sex. Although the ketamine + olanzapine groups showed a slight upward trend in BDNF levels (approximately 80–85% of control), this was not statistically significant (p > 0.05 vs. ketamine in both sexes). As expected, olanzapine-treated groups were not significantly different from controls (suggesting olanzapine alone had negligible effect on baseline BDNF).
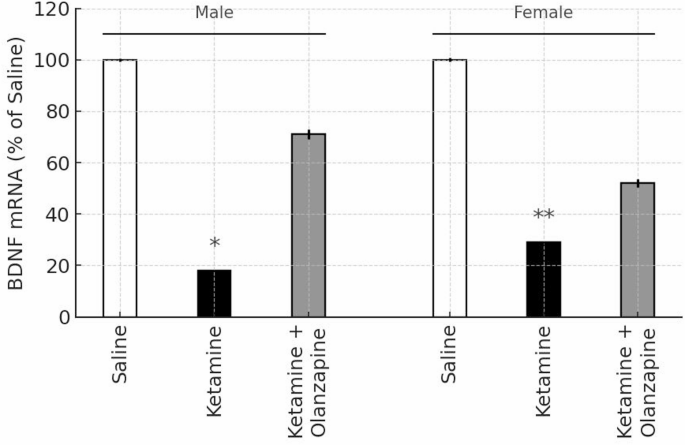
BDNF mRNA expression in the prefrontal cortex of male and female rats, expressed as percentage of same-sex saline control (100%). White, black, and gray bars represent the Saline, Ketamine, and Ketamine + Olanzapine groups, respectively. Bars show mean ± SE (n = 3 per group), and *p < 0.05, *p < 0.01 vs. same-sex saline controls. Note: The olanzapine-only group is not shown, as BDNF mRNA was not measured in that condition.
A two-way ANOVA (Treatment × Sex) was conducted including only the groups involved in BDNF measurement (saline, ketamine, ket + olanzapine; note that olanzapine-only was not measured for BDNF). This revealed a significant effect of Treatment (F2, 12 = 13.61, p < 0.001). Neither the main effect of Sex (F1, 12 = 0.59, p = 0.46) nor the interaction (F2, 12 = 0.41, p = 0.67) was significant. Thus, ketamine reduced PFC BDNF expression regardless of sex, and olanzapine did not significantly restore BDNF in either sex (Fig. 8). The lack of sex difference in BDNF is notable given the behavioral sex differences, suggesting that BDNF downregulation is a robust effect of ketamine in both males and females. It also implies that olanzapine’s behavioral benefits observed in this model were achieved without significant changes in cortical BDNF expression.
In summary, our results demonstrate that sub-chronic ketamine induces a schizophrenia-like phenotype in rats, characterized by hyperlocomotion, reduced exploratory behavior, cognitive impairment, and decreased BDNF, with females showing greater sensitivity in several behavioral domains (hyperlocomotion, anxiety-like behavior, nociception, antidepressant-like effect). Olanzapine effectively reversed most ketamine-induced behavioral changes (locomotion, climbing, memory impairment, and female-specific changes in rearing, pain threshold, and FST immobility). However, olanzapine had no significant effect on the ketamine-induced decrease in BDNF expression, indicating that its therapeutic effects were likely mediated via other pathways.