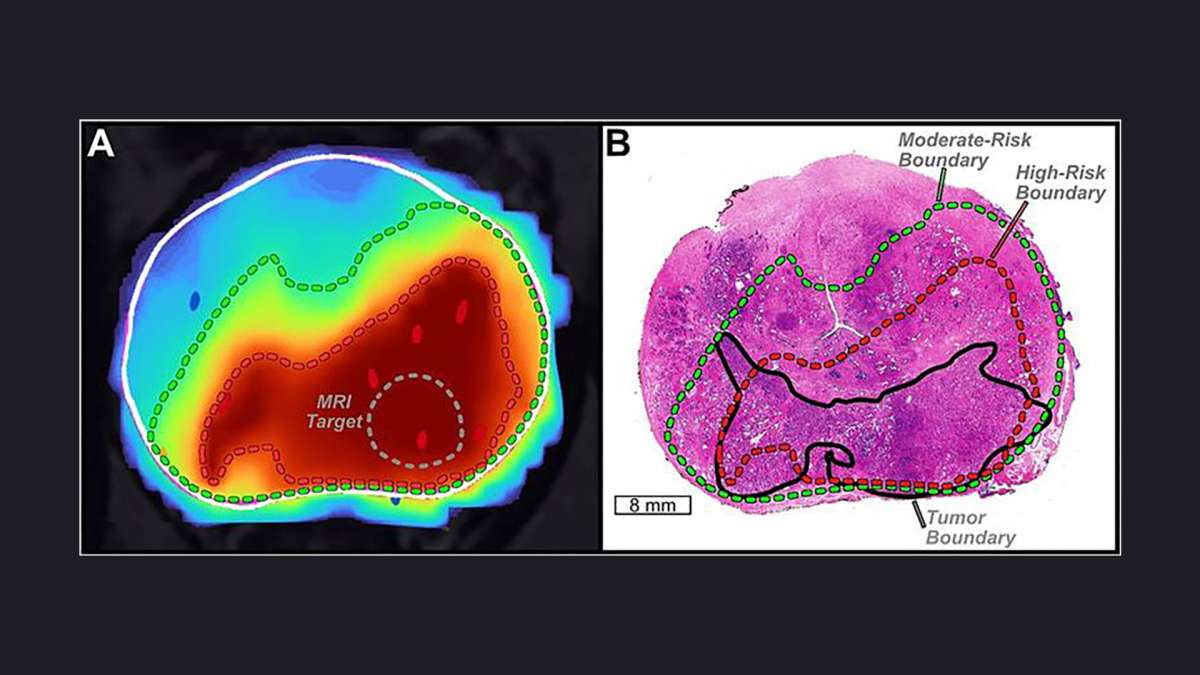
For men facing prostate cancer, knowing exactly where the disease lies can make the difference between a targeted treatment and a more invasive procedure. At UCLA Health, researchers are harnessing artificial intelligence to map tumors…
AI is helping doctors see prostate cancer more clearly and guide more effective treatments