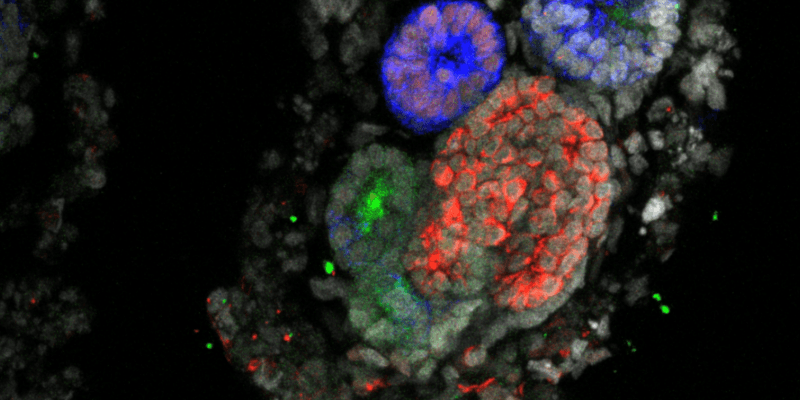
Animal and cell culture models have fallen short in kidney disease research. Kidney organoids may offer a better way forward.
Before he was a researcher, Ryuichi Nishinakamura was a nephrologist. In his clinic, he watched patients go into kidney failure again and again. He saw them become bed-bound and dependent on dialysis multiple times a week, their conditions worsening despite his best efforts. Eventually, all his patients passed away.
“I always remember each patient,” said Nishinakamura, now a kidney development researcher at Kumamoto University. “I was very, very frustrated. I couldn’t do anything.”
Ryuichi Nishinakamura has spent his career identifying kidney progenitor cells and growing kidney organoids.
Credit: Ryuichi Nishinakamura
That frustration drove him to study the kidney in greater depth. He identified key progenitor cells involved in the organ’s development and learned how to grow them in the lab. His work was pivotal to the creation of kidney organoids: in vitro model systems that mimic the functional units of the human kidney. These models are poised to transform drug discovery and disease research — and researchers like Nishinakamura have even bigger hopes for the future of organoids.
More than one in seven adults in the US suffer from chronic kidney disease, which ranks among the leading causes of death in the country (1). But until now, research on kidney disease has faced major hurdles. The kidney is a highly complex organ composed of numerous specialized cell types, making it difficult to replicate using traditional cell culture. And while animal models have played an important role in investigating human kidney diseases, these models fall short for several reasons (2).
For one, genetic differences play a big role in human kidney disorders — variation that inbred animal models lack. Severe cases tend to occur in older adults, whereas most laboratory animals are typically young, free of the comorbidities that influence kidney health. And physiological differences between species mean that animal models aren’t always reliable predictors of drug toxicity (3).
In the last decade, kidney organoids have emerged as promising alternatives. These models are a simplified version of a nephron, the functional unit of the kidney, and they’re composed of multiple different cell types arranged in a three-dimensional structure resembling the architecture of the organ.
“These organoids offer a unique opportunity to investigate a wide range of research questions in a setting that closely mirrors the in vivo environment,” said Joost Hoenderop, a kidney physiology researcher at Radboud University Medical Center. “It is incredibly fascinating to watch these kidney organoids grow on the microscope. As the structures emerge, with their distinct nephron segments, it feels like observing a work of art in motion.”
Modeling disease in a dish
To recreate diseases in an organoid model, researchers can edit specific genes or even grow organoids from cells derived from patients with a specific condition. Currently, a major focus area is polycystic kidney disease (PKD), a genetic disorder that affects about 500,000 people in the US (4). It causes clusters of cysts to grow in the kidney, as well as throughout the rest of the body, eventually choking out healthy tissue and damaging the kidneys.
It’s been known for years that PKD is caused by mutations in the PKD1 and PKD2 genes, but treatment options have been limited. That’s partially because there’s been no in vitro models that replicate PKD, and mouse models have major shortcomings.
“It seems like it’s easier to treat PKD in a mouse than it is in a person,” explained Benjamin Freedman, a kidney disease researcher at the University of Washington. In preclinical studies, drugs that performed well in mouse models often failed in human trials.
“Animal models are mechanistically complex, so they’re hard to really get into and perturb and figure out exactly how a pathway is working,” Freedman said. “Organoids are a little better in that regard. You can watch them. You can take things out of the system and see if they’re necessary or change components of the system in a way that’s more difficult to do in a living organism.”
Using this method, Freedman uncovered potential new ways to treat PKD. He found that a compound that inhibits myosin, a molecule involved in the contraction of non-muscle tissues, made cysts even worse. The results suggest that when myosin fails to keep tubules in the kidney contracted, those tissues blow up like water balloons and form cysts (5).
I don’t think the animal models are going to disappear anytime soon. But I think we’ll see, increasingly, a choice presented between using animals and using organoids.
– Benjamin Freedman, University of Washington
“That’s leading to different types of directions for therapeutics that could intervene and activate myosin,” he continued. “The idea is that if we can get the balance of myosin contractility right, then we can prevent the cysts from forming throughout the body.”
Working with his company, Plurexa, Freedman has already identified several candidate molecules that target myosin in their organoid model. These are now being further elucidated using both traditional in vitro methods and animal models.
Going forward, Freedman thinks we’ll see more drug research taking this hybrid approach.
“There is a strong general interest in moving organoids to front and center for drug development,” he said. “I don’t think the animal models are going to disappear anytime soon. But I think we’ll see, increasingly, a choice presented between using animals and using organoids.”
A work in progress
Still, kidney organoids are far from perfect.
“Although they are beautiful, they still are relatively primitive compared to an actual functional organ,” Freedman admitted.
Organoids can’t yet produce urine — an essential feature of the kidney. They lack blood vessels and they’re missing some of the higher-order structures that are present in a real kidney. Reproducibility is a challenge, too, as the varied cell types that comprise kidney organoids may develop slightly differently each time they’re cultured.
To avoid these limitations, researchers are building even more precise model systems that focus on single cell types. Andrew McMahon, a stem cell biology and regenerative medicine researcher at the University of Southern California, is making headway on these more high-fidelity, specialized models.
“The organoid approach tries to generate diversity and complexity that mirrors the organization of the normal kidney,” McMahon said. “And in some ways, I think that that’s great, but it may be too complex.”
Less complexity, or more?
McMahon believes that drug research has just as much to learn from simplified models as it does from complex organoids. He points to proximal tubule cells as an example. These are a type of cell in the kidney that reabsorb nutrients such as glucose and filter toxins, making them a key target for drug research. While proximal tubule cells are represented in kidney organoids, their full function may not be replicated in a real kidney. A simplified system could allow for faster and more efficient disease modeling and drug screening.
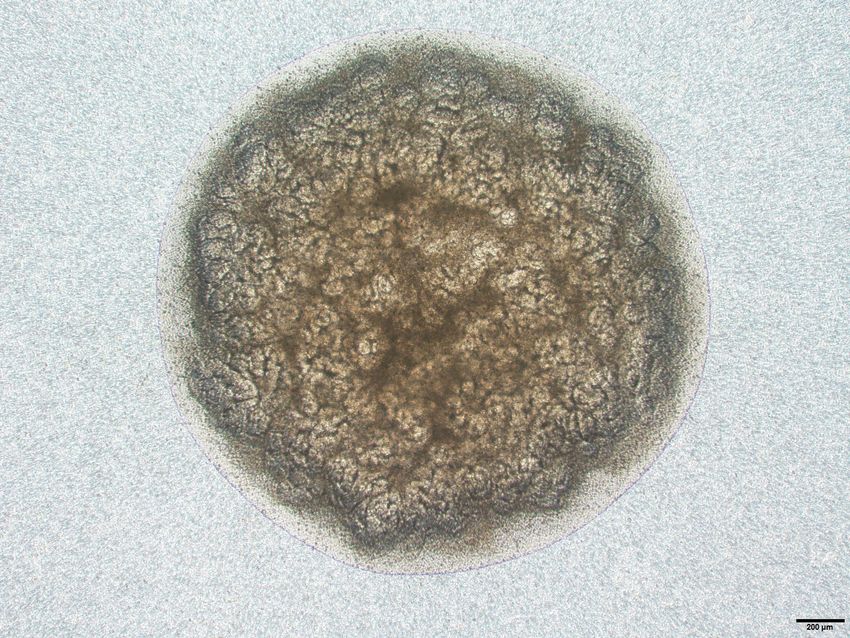
Ryuichi Nishinakamura and his team develop kidney organoids.
Credit: Ryuichi Nishinakamura
Others are adopting the opposite approach, attempting to make kidney organoids more true-to-life by enhancing their complexity.
“One of our long-term goals is to culture fully-functional organoids that can effectively mimic nephron function,” said Hoenderop. His lab ultimately hopes to create tissue that restores function in people with kidney damage. Some labs are going further, working to engineer a transplantable kidney.
The kidney is often described as the second most complex organ in the body after the brain. Its formation involves three types of progenitor cells migrating from disparate locations at different stages of fetal development (6). It’s a complex choreography, aimed at one day creating transplantable, fully functional organs for people with kidney disease, a goal Nishinakamura continues to pursue.
“We’re working very hard to combine all of these three progenitors to make the whole kidney,” he said. His lab is also developing urethral tissue and working to mature the cell types further. Currently, kidney organoid cells are immature, meaning they don’t resemble adult kidney cells and can only be used to study diseases that are present from very early development.
There is a long road ahead, but Nishinakamura remains hopeful.
“I didn’t expect that I could do this in my life,” he said. “Initially, generating kidney organoids was thought to be impossible.
“I always tell my graduate students, ‘Don’t say impossible.’ Everything can be possible.”
References
- National Institute of Diabetes and Digestive and Kidney Diseases. Kidney Disease Statistics for the United States (2024).
- Liang, J. & Liu, Y. Animal Models of Kidney Disease: Challenges and Perspectives. Kidney360 4, 1479-1493 (2023).
- Van Norman, G.A. Limitations of Animal Studies for Predicting Toxicity in Clinical Trials: Is it Time to Rethink Our Current Approach?J Am Coll Cardiol Basic Transl Sci 4, 845-854 (2019).
- Mahboob, M. et al. Autosomal Dominant Polycystic Kidney Disease. 2024.
- Czerniecki, S.M. et al. High-Throughput Screening Enhances Kidney Organoid Differentiation from Human Pluripotent Stem Cells and Enables Automated Multidimensional Phenotyping. Cell Stem Cell 22, 929-940.e4 (2018).
- Huang, B. et al. Epigenetic regulation of kidney progenitor cells. Stem Cells Transl Med. 9, 655-660 (2020).