The egg hatching rate
Eggs of Ae. aegypti and Ae. albopictus hatched across all tested temperatures ranging from 15 °C to 40 °C (Fig. 1a; Table 1). Hatching rates were significantly influenced by temperature (two-way ANOVA, F(5,23) = 80.65, P < 0.0001) and species (two-way ANOVA, F(1,23) = 9.530, P = 0.005), but no significant interaction was observed between these factors (two-way ANOVA, F(5,23) = 0.566, P > 0.05) (Additional file 3: Table S1). The highest hatching rates were recorded at 20 °C, with an average of 98.50 ± 0.29% for Ae. aegypti and 90.63 ± 0.88% for Ae. albopictus (Table 1). In contrast, the lowest rates were observed at 40 °C, averaging 5.26 ± 0.92% for Ae. aegypti and < 1% (0.83 ± 0.33%) for Ae. albopictus. We also found that Ae. aegypti had significantly higher hatching rates than Ae. albopictus at 20 °C, 25 °C and 40 °C (t-test, t = 4.51–8.52, df = 2, P < 0.05), whereas no significant differences were observed at 15 °C, 30 °C and 35 °C (Fig 1a t-test, t = 0.33–3.29, df = 2, P > 0.05). Statistical analysis revealed a significantly higher hatching rate at lower (15–30 °C) than higher temperatures (35 and 40 °C) for both Ae. aegypti (ANOVA, F(5,12) = 61.92, P < 0.0001) and Ae. albopictus (ANOVA, F(5,11) = 28.09, P < 0.0001), and no significant differences were observed among 15, 20, 25 and 30 °C.
a Hatching success, b larval survival rate, c pupal survival rate, d wing length, e blood-feeding rate, f egg-laying of female Aedes aegypti and Aedes albopictus mosquitoes at different temperatures. Lines represent mean ± standard error (SE). Statistical comparisons between species at each temperature were performed using Student’s t-test with significance accepted at ns: p > 0.05 (ns), *p < 0.05, **p < 0.01, ***p < 0.001 and ****p < 0.0001
Larval survival rate
Temperature significantly influenced the survival rate of larvae (two-way ANOVA, F(5,23) = 108.07, P < 0.0001), but no significant differences were found between species (Fig. 1b, two-way ANOVA, F(1,23) = 0.001, P > 0.05), and there was no interaction between these factors (two-way ANOVA, F(5,23) = 0.230, P > 0.05) (Additional file 3: Table S1). Larval survival exceeded > 87% for both species at 15, 20, 25 and 30 °C (Fig. 1b; Table 1). The highest larval survival rate was observed at 25 °C for Ae. aegypti (97.45 ± 1.78%) and 20 °C for Ae. albopictus (94.09 ± 2.88%). In contrast, the lowest larval survival rate was observed at 40 °C for both species, where larvae failed to survive beyond the first instar. In addition, our results revealed a significantly higher larval survival rate at lower temperatures (15–30 °C) compared to the two highest temperature treatments (35 and 40 °C) for both Ae. aegypti (ANOVA, F(5,12) = 79.89, P < 0.0001) and Ae. albopictus (ANOVA, F(5,11) = 39.33, P < 0.0001), and no significant differences were observed among 15, 20, 25 and 30 °C.
Pupal survival rate
Pupal survival rate was significantly influenced by temperature (two-way ANOVA, F(4,19) = 3.58, P = 0.025), but no significant differences were found between the species (Fig. 1c, two-way ANOVA, F(1,19) = 0.2, P > 0.05) and no interaction between the temperature and species (two-way ANOVA, F(4,19) = 0.23, P > 0.05) (Additional file 3: Table S1). The highest pupal survival was recorded at 25 °C for both species, with 96.82 ± 2.13% for Ae. aegypti and 96.84 ± 0.95% for Ae. albopictus. In contrast, the lowest survival rate was observed at 35 °C, with 82.98 ± 8.66% for Ae. aegypti and 78.45 ± 7.54% for Ae. albopictus (Fig. 1c; Table 1). Statistical analysis indicated a significantly higher pupal survival rate for both species at 20 and 25 °C compared to 35 °C (ANOVA, F(4,23) = 4.27, P = 0.01).
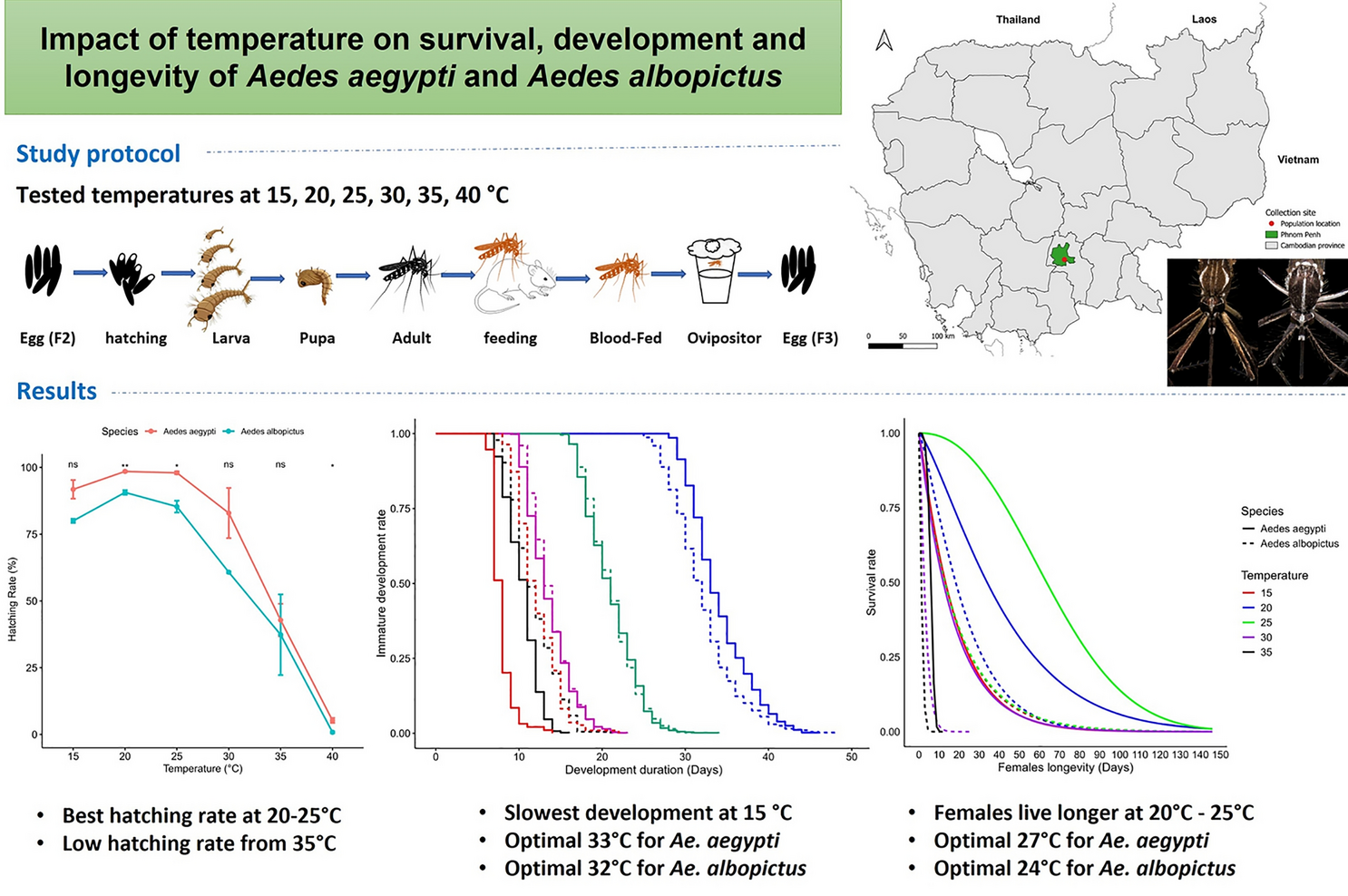
Immature development duration
The development duration of immature stages (from egg to adult emergence) varied significantly across the tested temperature range (15–35 °C) (two-way ANOVA, F(5,23) = 96.6, P < 0.0001) and between species (two-way ANOVA, F(1,23) = 6.42, P = 0.01), and significant interaction was observed between these factors (two-way ANOVA, F(4,19) = 9.93, P = 0.0001). Weibull’s model highlighted that the shortest development duration for Ae. aegypti occurred at 35 °C (7.83 days), while Ae. albopictus exhibited its shortest development time at 30 °C (11.80 days). In contrast, the development duration decreased with increasing temperature, with the longest durations observed at 15 °C, with 34 days for Ae. aegypti and 32 days for Ae. albopictus. Interestingly, some individuals in the final immature stages took longer at 15 °C, with Ae. aegypti spending up to 46 days and Ae. albopictus up to 44 days (Fig. 2, Table 2). We also found no statistical difference between the two species at all tested temperatures (t-test, t = – 2.84–1.82, df = 2–3, P > 0.05), except at 35 °C, where Ae. aegypti developed significantly faster than Ae. albopictus (t-test, t = – 5.33, df = 2, P = 0.02), with 7.83 days and 12.2 days, respectively.
Developmental duration of immature stages of Aedes aegypti and Aedes albopictus across different temperatures
Differences in the duration of immature development were attributed to varying rates of progression through each immature stage, including egg hatching, larval development and pupation. During larval stage, the development duration was significantly influenced by temperature (two-way ANOVA, F(4,19) = 509.98, P < 0.0001) between the two species (two-way ANOVA, F(1,19) = 5.74, P < 0.0001, P > 0.05). A significant interaction between the temperature and species was also observed (two-way ANOVA, F(4,19) = 13.94, P < 0.0001). The longest larval development duration was recorded at 15 °C for both species, averaging 25.5 days for Ae. aegypti and 23.5 days for Ae. albopictus. Additionally, the last individual larvae took the longest to develop at 15 °C, with a maximum duration of 38 days for Ae. aegypti and 44 days for Ae. albopictus (Additional file 1: Fig. S1a). In contrast, the shortest larval development durations were observed at 35 °C for Ae. aegypti and 30 °C (9.92 days) for Ae. albopictus. In particular, Ae. aegypti larvae developed signifyingly faster than Ae. albopictus at 30 (t-test, t = – 5.98, df = 2, P = 0.01) and 35 °C (t-test, t = – 8.03, df = 2, P = 0.009), while no significant differences were observed at 15, 20 and 25 °C (Additional file 1: Fig. S1b, Table 2, t-test, t = – 0.97–2.06, df = 2, P > 0.05).
During pupal stage, the development duration was significantly influenced by temperature (two-way ANOVA, F(4,19) = 98.91, P < 0.0001), but no significant differences were found between species (two-way ANOVA, F(1,19) = 2.26, P > 0.05), and there was no interaction between these factors (two-way ANOVA, F(4,19) = 0.8, P > 0.05). Similar to larvae, the longest duration of pupal development duration was recorded at 15 °C for both species, averaging 9.29 (maximum 14) days for Ae. aegypti and 9.35 (maximum 21) days for Ae. albopictus. In contrast, the fastest development duration was recorded at 35 °C for both species, averaging 1.97 (maximum 7) days for Ae. aegypti and 2.01 days for Ae. albopictus (maximum 13 days) (Table 2, Additional file 1: Fig. S1c). However, no significant differences were observed between the two species at any temperature (Table 2, Additional file 1: Fig. S1d, t-test, t = – 2.52–0.67, df = 1–3, P > 0.05).
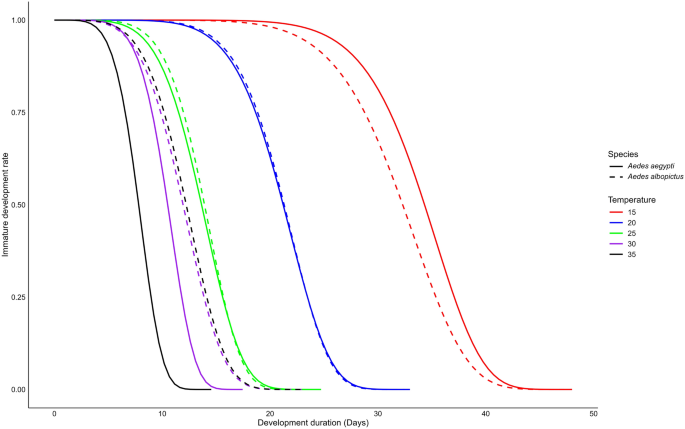
Adult male and female longevity
We found that female longevity for each species was significantly strongly affected by temperature (two-way ANOVA, F(4,19) = 34.91, P < 0.0001) and species (two-way ANOVA, F(1,19) = 48.08, P < 0.0001). Additionally, a significant interaction between temperature and species was observed (two-way ANOVA, F(4,19) = 12.08, P < 0.0001). Weibull’s model estimated that Ae. aegypti females had the longest survival at 25 °C, with an average lifespan of 66.7 days and a maximum of 122 days. However, the last surviving Ae. aegypti female lived up to 146 days at 20 °C. Regarding Ae. albopictus females, the longest survival was observed at 20 °C, with an average lifespan of 22.6 days and a maximum of 99 days. In contrast, the shortest female lifespan was recorded at 35 °C for both species, averaging 5.86 (maximum 8) days for Ae. aegypti and 1.41 (maximum 8) days for Ae. albopictus (Fig. 3; Table 2, Additional file 2: Fig. S2a). We also found that female longevity was significantly longer at 20 °C and 25 °C compared to 15 °C, 30 °C and 35 °C for both Ae. aegypti (ANOVA, F(4,10) = 41.53, P < 0.0001) and Ae. albopictus (ANOVA, F(4,9) = 41.53, P = 0.01). Between the two species, Ae. aegypti females lived significantly longer than Ae. albopictus females at 25, 30 and 35 °C (t-test, t = 5.42–12.4, df = 2–3, P < 0.05). However, no significant difference was observed at 15 °C and 20 °C (Additional file 2: Fig. S2b, t-test, t = −0.02–2.40, df = 2, P > 0.05).
Longevity of female Aedes aegypti and Aedes albopictus mosquitoes reared at different temperatures
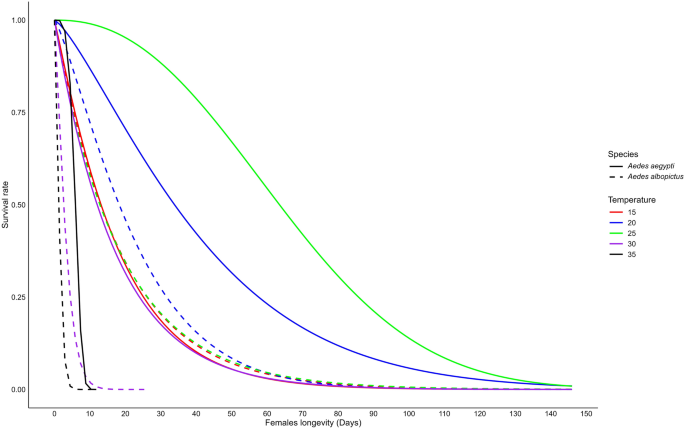
Our results show a significant difference in longevity between male and female mosquitoes, with females having notably longer lifespans for both Ae. aegypti species (t-test, t = 2.39, df = 16, P = 0.02) and Ae. albopictus (t-test, t = 2.38, df = 16, P = 0.03). We found that male longevity for each species was significantly influenced by temperature (two-way ANOVA, F(4,19) = 12.32, P < 0.0001) and species (two-way ANOVA, F(1,19) = 47.24, P < 0.0001). Additionally, a significant interaction between temperature and species was observed (two-way ANOVA, F(4,19) = 5.00, P = 0.006). Weibull’s model estimated the longest surviving of males was at 25 °C for both species, averaging 23.6 (maximum 108) days for Ae. aegypti and 9.43 (maximum 63) days for Ae. albopictus. In contrast, the shortest male lifespan was recorded at 35 °C for both species, averaging 6.14 (maximum 9) days for Ae. aegypti and 2.46 (maximum 13) days for Ae. albopictus (Fig. 4; Table 2, Additional file 2: Fig. S2c). We also found that male longevity was significantly longer at 20 °C and 25 °C compared to 15 °C, 30 °C and 35 °C for Ae. aegypti (ANOVA, F(4,10) = 31.27, P < 0.0001), but no significant difference was recorded for Ae. albopictus (ANOVA, F(4,9) = 0.85, P > 0.05). Between the two species, Ae. aegypti males lived significantly longer than Ae. albopictus females at 20, 25 and 30 °C (t-test, t = 5.45–9.07, df = 2–3, P < 0.05). However, no significant difference was observed at 15 °C and 35 °C (Additional file 2: Fig. S2d, t-test, t = 0.44–1.16, df = 2, P > 0.05).
Longevity of male Aedes aegypti and Aedes albopictus mosquitoes reared at different temperatures
Temperature thresholds
The development rate during the immature stage of Ae. aegypti and Ae. albopictus followed a non-linear pattern with temperature, best described by the Logan regression model (Fig. 5). The estimated optimal temperature (Topt) was 33.33 °C for Ae. aegypti and 32.31 °C for Ae. albopictus, with development rates declining at both lower and higher temperatures. The upper thermal limit (Tmax) was 40.0 °C for both species, while the lower threshold (Tmin) was approximated at 5 °C (Fig. 5). The survival of female Ae. aegypti and Ae. albopictus followed a quadratic trend model with temperature (Fig. 6). The estimated optimal temperature (Topt) was 27.06 °C for Ae. aegypti and 24.54 °C for Ae. albopictus, with survival declining at both lower and higher temperatures. The lower (Tmin) and upper (Tmax) thermal limits were 14.97–39.15 °C for Ae. aegypti and 11.02–38.07 °C for Ae. albopictus (Fig. 6).
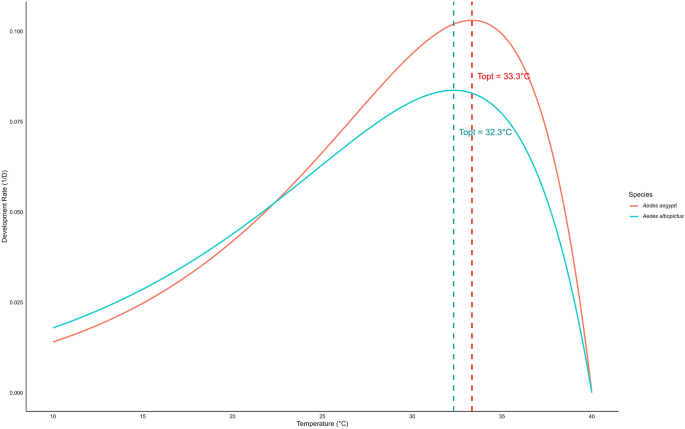
Estimated optimal, upper and lower temperature thresholds for development rates of immature stages in Aedes aegypti and Aedes albopictus. The optimal temperature indicates the peak development rate, and the lower and upper thresholds represent the temperature limits where the development rate drops to zero
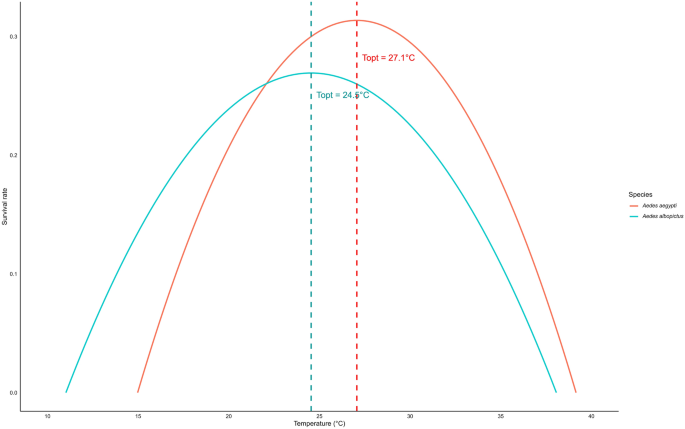
Estimated optimal, upper and lower temperatures for survival of Aedes aegypti and Aedes albopictus females. The optimal temperature indicates the peak survival rate, and the lower and upper thresholds represent the temperature limits where the survival rate drops to zero
Body size of female mosquitoes
Body size of mosquitoes was indicated by the length of the female’s wings for each species. The wing length of Ae. aegypti and Ae. albopictus varied significantly across the tested temperatures (two-way ANOVA, F(4,750) = 404.35, P < 0.0001) and between species (two-way ANOVA, F(1,750) = 49.37, P < 0.0001); significant interaction was also observed between these factors (Fig. 1d; Table 1, two-way ANOVA, F(4,750) = 18.55, P < 0.0001) (Additional file 3: Table S1). A clear trend was observed, with wing length increasing as the temperature decreased. For Ae. aegypti, the longest average wing length was observed at 15 °C (3.15 ± 0.01 mm), while the shortest was at 35 °C (2.42 ± 0.02 mm). Similarly, for Ae. albopictus, the longest average wing length occurred at 15 °C (2.92 ± 0.01 mm), and the shortest was at 35 °C (2.24 ± 0.02 mm) (Fig. 1c; Table 1). Statistical analysis revealed Ae. aegypti had significantly longer wings than Ae. albopictus at 15 °C and 35 °C (t-test, t = 5.45–12.4, df = 67–177, P < 0.05), but no significant difference was observed between the two species at 20 °C, 25 °C and 30 °C (t-test, t = – 0.44–1.66, df = 136–177, P > 0.05).
Blood-feeding rate
Blood-feeding rates of female mosquitoes were assessed during two feeding trials conducted at temperatures ranging from 15 °C to 30 °C, excluding 35 °C because of high adult mortality before the feeding day. The average number of blood-fed females was significantly influenced by temperature (two-way ANOVA, F(3,37) = 19.56, P < 0.0001), but no significant differences were found between the species (Fig. 1d, two-way ANOVA, F(1,37) = 0.34, P > 0.05) and no interaction between the species (two-way ANOVA, F(3,37) = 0.52, P > 0.05) (Additional file 3: Table S1). The highest feeding rate was observed at 30 °C for Ae. aegypti (61.02 ± 4.43%) and 25 °C for Ae. albopictus (52.46 ± 17.88%) (Fig. 1e; Table 1). In contrast, the lowest blood-feeding rates were recorded at 15 °C for both species, where Ae. aegypti females did not feed at all and only three Ae. albopictus individuals fed (0.88 ± 0.88%). We also found that the blood-feeding rate of both species was not significantly different at all tested temperatures (15–35 °C) (t-test, t = – 0.59–0.46, df = 3–9, P > 0.05).
Number of eggs laid
Our results indicate that the average number of eggs laid per female mosquito varied significantly across the tested temperature range (15–30 °C) (two-way ANOVA, F(3,156) = 42.39, P < 0.0001) and between the two species (two-way ANOVA, F(1,156) = 49.02, P < 0.0001), and there was a significant interaction between these factors (two-way ANOVA, F(2,156) = 16.95, P < 0.0001) (Additional file 3: Table S1). The highest egg production was recorded at 30 °C for Ae. aegypti, with an average of 64.03 ± 4.37 eggs per female, and at 25 °C for Ae. albopictus, with an average of 14.96 ± 4.76 eggs per female. In contract, the lowest egg reproduction was recorded at 15 °C, where three Ae. albopictus individuals that fed on blood did not lay any eggs (0.00 ± 0.00 eggs/female). For Ae. aegypti, egg production at 15 °C was unknown, as no females fed on blood at this temperature. We also found that Ae. aegypti females produced significantly more eggs than Ae. albopictus at 25 and 30 °C (t-test, t = 4.89–4.79, df = 12–50, P < 0.05) (Fig. 1f; Table 1).