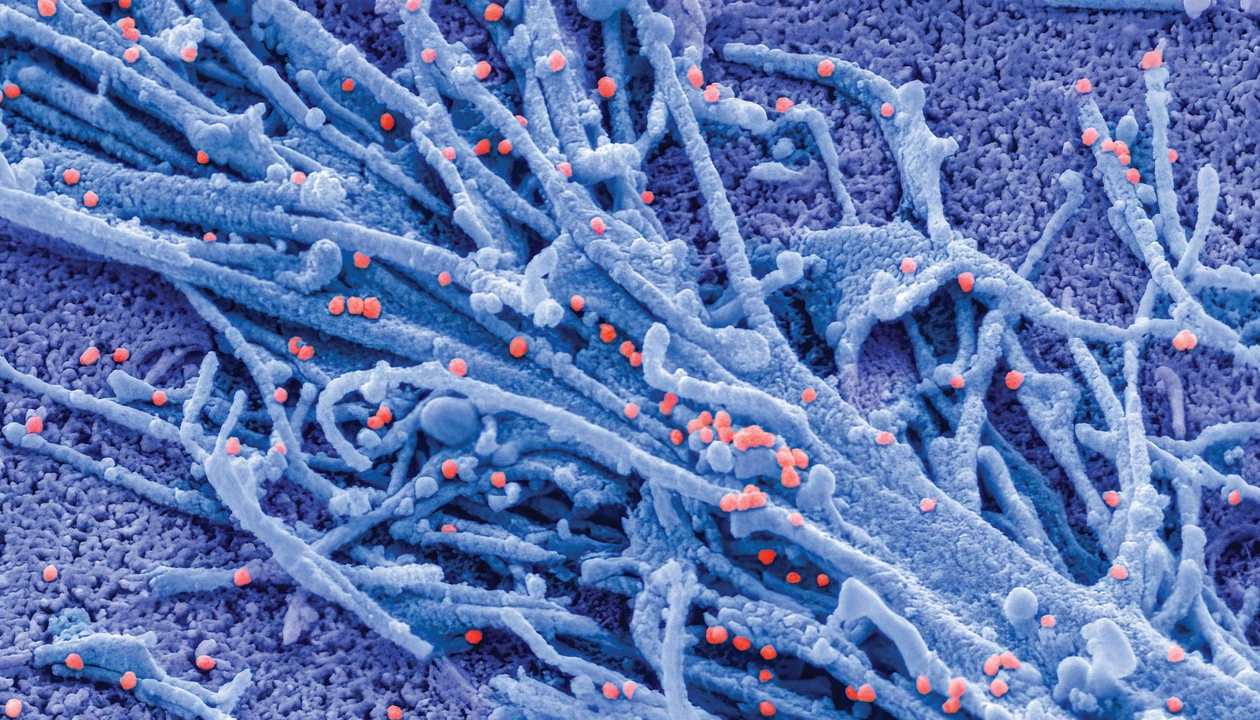
Researchers have made a broad-spectrum antiviral that works against several virus families by blocking N-glycans—long thought of as undruggable targets (Sci. Adv. 2025, DOI: 10.1126/sciadv.ady3554).
The branched sugars are ubiquitous in cells, decorating proteins to mediate numerous biological functions. As a result, their dysregulation can cause disease. Adam Braunschweig at the Advanced Science Research Center at the CUNY Graduate Center and colleagues initially set out to develop glycan inhibitors to control cellular processes involved in ailments like cancer. But they soon realized that viruses, which use the sugars to bind to host cells, would be the perfect proof of concept for the compounds.
The approach differs from other attempts to block glycans because it largely ignores a common molecular binding principle developed by Nobel Prize–winner Donald J. Cram: that host molecules should be rigid and preorganized so they don’t pay an entropic penalty that makes binding less favorable.
“I didn’t do my PhD in carbohydrate chemistry, which is probably the only reason why I decided to go into it. I was ignorant,” Braunschweig says. But not having preconceived notions allowed Braunschweig to explore flexible organizations for synthetic carbohydrate receptors (SCRs) that take the entropic penalty when they glom on to glycans.
When sugars differ only by the orientation of only a couple of hydroxyl groups, rigid inhibitors struggle to bind, but flexibility allows the SCRs to adjust their shapes to a sugar’s unique hydroxyl pattern. The receptors have a core made of two bound aromatic rings, with four extended arms that rotate and interact with hydroxyl and hydrogen groups on the sugars. This mimics natural sugar-binding proteins, which use many weak interactions to achieve strong bonds.
In a screen of 57 different SCRs against six replication-defective enveloped viruses from three different viral families and a nonenveloped glycosylated rotavirus, two candidates, SCR005 and SCR007, were found to reduce all virus levels.
In mice, the two compounds didn’t have toxic effects even at high doses, and when the researchers gave the compounds to mice infected with SARS-CoV-2, they reduced mortality and disease severity.
The researchers were able to show that their most promising candidate, SCR007, directly binds N-glycans and that it selectively binds fucosylated N-glycans that have three or four branches—an organization common in viruses. But Braunschweig says the team also found some evidence that the SCRs may be affecting cellular function independent of virus infection, and it will take more research to figure out exactly what’s going on.
Reuben Harris, an antiviral expert at the University of Texas Health Science Center at San Antonio who was not involved in the work, says that important next steps will be to determine whether this class of antivirals could create more-pathogenic variants through resistance mechanisms, and verify that the compounds don’t interfere with sugars on immune cells. Nevertheless, he says the compounds could be “pretty powerful” if they can create a therapeutic window with manageable side effects.
For now, Braunschweig and his colleagues are preparing for Phase 1 clinical trials with hopes to explore SCRs for treatment of other diseases, such as cancer.
Chemical & Engineering News
ISSN 0009-2347
Copyright ©
2025 American Chemical Society