Microglia safeguard the proliferation and survival of young GABAergic interneurons by secreting insulin-like growth factor 1 (IGF-1), according to a new study of human brain tissue and organoids. The finding points to the potential origin of the brain signaling imbalance implicated in autism and other conditions.
Microglia contribute to brain development, past findings show, but their exact function has been unclear. Some experiments showed that these cells prune neural circuits, but later work called that idea into question.
The new research “identifies microglia as really an important source of IGF, and one that sets the supply of GABAergic interneurons in the developing brain,” says Damon Page, principal investigator at Seattle Children’s Research Institute. Page was not involved in this work but led an earlier investigation that showed IGF-1 prevents microcephaly in a mouse model of autism when administered during a critical window soon after birth.
This new study “extends back that window into the embryonic period,” he says, with implications for understanding both typical development and conditions such as autism. The study was published 6 August in Nature.
T
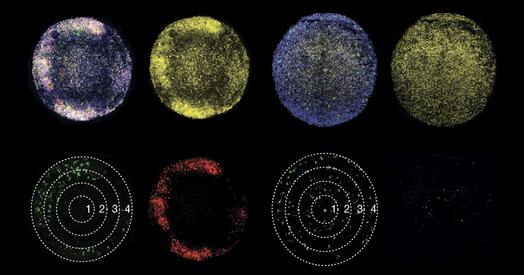
he investigators used staining techniques to pinpoint microglia in the medial ganglionic eminence, where interneurons form, in human brain tissue samples at various developmental stages. At early developmental stages, microglia were sprinkled throughout brain matter, but later on these cells arranged themselves around clusters of GABAergic neuroblasts, with their processes extending into the clusters. Microglia also aligned themselves with radial glia, the precursors to many brain cells.
Based on existing data, IGF-1 emerged as the chemical most likely to mediate microglia’s effects on developing cell types, and in organoid models of the developing human brain, the cells secreted IGF-1, they found.
And organoids that lacked typical microglia, or that contained microglia lacking IGF-1, developed significantly fewer GABAergic interneurons. Treating the IGF-1 knockout organoids with IGF restored typical proliferation.
Knocking out microglial IGF-1 in the medial ganglionic eminence of developing mouse embryos did not appear to affect the proliferation of interneurons. That result was the most surprising, says study investigator Xianhua Piao, professor of pediatrics at the University of California, San Francisco. But humans have many more interneurons than mice do, so perhaps the microglial IGF-1 mechanism is specific to humans, she says.
Severe infection during pregnancy increases the chances of having a child with autism, and the findings suggest a mechanism via a gene-environmental interaction, says Piao, who is also a practicing neonatologist.
Inflammation, an environmental insult, could hamper how microglia foster GABAergic interneuron development, but perhaps only when there are multiple genetic hits, she hypothesizes. Her lab plans to introduce inflammation into organoids to see how microglia and IGF-1 pathways respond.
Infections and other forms of inflammation during pregnancy can be difficult to prevent, so IGF-1 offers a hopeful avenue for treatment, says Marie-Ève Tremblay, professor of neurobiology at the University of Victoria in Canada, who was not involved in the research. “If there’s ways to keep microglia healthy or rescue them once they have been compromised, I think this would be groundbreaking.” A synthetic form of IGF-1, trofinetide, is already approved by the U.S. Food and Drug Administration to treat Rett syndrome.
The new work raises an important question for future research, Tremblay says: whether microglia nurture GABAergic interneuron development differently in males and females. “Most immune mechanisms differ between sexes,” she says. “If there’s a difference there, it could also help to understand why autism has this sexual dimorphism.”