Tirofiban, as a GP IIb/IIIa receptor antagonist, competitively inhibits the bridging of platelets via fibrin, which is the final pathway of platelet aggregation. This mechanism renders it a crucial agent in the prevention of thrombus formation during intravascular interventional therapies for atherosclerotic cardiovascular and cerebrovascular diseases. Consequently, it is of importance to monitor the adverse drug reactions associated with tirofiban in clinical practice. This study, leveraging the FAERS database, aims to comprehensively evaluate the real-world safety of tirofiban by analyzing the signal strength of reported AEs.
Bleeding, the most common adverse reaction to antiplatelet agents due to their inhibition of platelet function, aligns with the findings of our study. Hemorrhage was the most frequently reported PT (n=370), while vascular disorders (n=547) ranked second as the SOC term with the largest number of AEs, following cardiac disorders (n=862). The strong signals for cardiac-related PTs such as cardiac arrest, myocardial infarction should not be interpreted as adverse drug reactions, as they primarily represent the underlying cardiac conditions for which tirofiban is clinically indicated. Except from “hemorrhage”, positive PTs also involved bleeding classified into specific organ systems. Among these, 322 reports met the criteria for severe bleeding as defined by the Thrombolysis in Myocardial Infarction (TIMI) clinical trial, including 300 reports of intracranial hemorrhage (PTs: cerebral hemorrhage, hemorrhage intracranial, hemorrhagic stroke, intracranial hematoma, subarachnoid hemorrhage and intraventricular hemorrhage), 16 reports of retroperitoneal hemorrhage (PTs: retroperitoneal hematoma, retroperitoneal hemorrhage), 13reports of hemorrhagic shock (PTs: shock hemorrhagic, hypovolemic shock) and 6reports of cardiac tamponade. Intracranial hemorrhage was the most frequently reported among all classified bleeding events, followed by gastrointestinal hemorrhage (n=29). The large number of intracranial hemorrhage may be related to the application of tirofiban in IS. However, since the majority of bleeding reports were not stratified, this comparison may lack practical significance.
In the treatment of ischemic stroke (IS), the pathophysiological characteristic that cerebral ischemia can easily transform into cerebral hemorrhage presents a challenge in balancing the antithrombotic efficacy of antiplatelet agents against the risk of bleeding14. In the saTIS trial, tirofiban, when compared to a placebo, did not increase the incidence of intracranial hemorrhage, which demonstrated the safety of tirofiban in the treatment of IS and laid the foundation for a series of subsequent randomized controlled trials15. Recent RESCUE BT trial indicated that tirofiban did not significantly increase the risk of intracranial hemorrhage in IS patients compared to a placebo12, and in the RESCUE BT2 trial, the incidence of tirofiban-induced intracranial hemorrhage was only 1%12,13. However, despite the safety of tirofiban having been confirmed by these trials, the RESCUE BT trial failed to establish the efficacy of tirofiban in IS patients undergoing cerebrovascular therapy. The application of tirofiban in the perioperative period of cerebrovascular endovascular treatment still lacks large-scale clinical evidence. In contrast, PCI has been developed earlier, and the use of tirofiban as an adjunctive medication during the perioperative period of PCI has accumulated a substantial amount of clinical evidence, with its safety having been verified in numerous clinical studies and practical applications. The PRISM-PLUS study showed no statistical difference in bleeding between tirofiban combined with heparin and heparin therapy alone. In this study, the incidence of tirofiban-induced major bleeding according to TIMI classification was 1.4%, and the incidence of minor bleeding was 0.5%.4
The second-ranked PT was thrombocytopenia (n=248), which is a common adverse reaction listed in the drug label of tirofiban. Clinical reports indicated that the incidence of thrombocytopenia induced by tirofiban ranges from 0.4% to 5.6%16. The specific cause of tirofiban-induced thrombocytopenia remains unclear, but it may be related to increased immune-mediated platelet destruction17. Although thrombocytopenia caused by tirofiban is often self-limiting, its occurrence is closely associated with severe bleeding and ischemic events18.
It is noteworthy that among all tirofiban-related AEs, the number of acute respiratory failure reports reached 95. Additionally, there were 20 reports of hemoptysis, 19 of pulmonary alveolar hemorrhage, 10 of pulmonary hemorrhage, and 4 of lung infiltration. In most patients treated with tirofiban, the occurrence of respiratory failure may be related to the primary cardiac condition, such as ACS associated cardiac dysfunction, which can lead to pulmonary congestion and edema, thereby affecting pulmonary ventilation and gas exchange functions. However, clinical reports have also indicated that diffuse pulmonary hemorrhage can cause severe respiratory failure19,20,21. Moreover, diffuse pulmonary hemorrhage can be exacerbated by an increase in pulmonary artery wedge pressure, which in this case could be caused by cardiac dysfunction. In a retrospective study, researchers analyzed 1020 patients undergoing PCI and treated with GP IIb/IIIa receptor antagonists, among whom 7 patients developed diffuse alveolar hemorrhage. Specifically, in patients treated with tirofiban, the incidence was 0.9%22. The relationship between tirofiban and decreased respiratory function warrants further clinical research.
Our study also reported potential adverse reactions not mentioned in the drug label for tirofiban. Firstly, there were severe bleeding types such as hemorrhagic shock (n=9), hemothorax (n=3), hypovolemic shock (n=4), which were also less commonly reported in clinical researches. Secondly, adverse events related to the coagulation system were observed, including prolonged APTT (n=5), coagulation disorders (n=5), and DIC (n=5). These may be partially associated with the concomitant use of anticoagulant drugs such as heparin. Thirdly, the occurrence of brain herniation (n=4) and cerebral edema (n=4) may be related to the cerebrovascular disease itself and changes in intracranial pressure caused by cerebral hemorrhage. Additionally, hemolysis (n=7) was reported, which may be associated with drug hypersensitivity reactions. On the other hand, adverse reactions that are listed in the drug label, such as nausea and fever, were also reported in small numbers in the FAERS database but did not meet the criteria for positive signal detection.
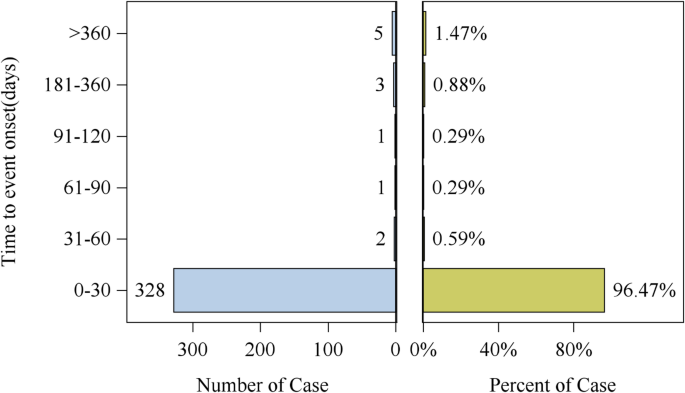
In terms of temporal analysis of the tirofiban-associated AEs, the median onset time was 1 day, and the vast majority of adverse events occurred within 1 month. This is consistent with the high affinity of intravenous tirofiban with platelet GP IIb/IIIa and thus rapid onset of antagonistic effect. Therefore, the monitoring of AEs should be initiated immediately after the drug administration, including both clinical symptom assessment (e.g., dyspnea in hemothorax and diffuse alveolar hemorrhage) and complete blood count evaluation. In atypical presentations of occult bleeding, decreasing hemoglobin levels may serve as an important diagnostic clue, while thrombocytopenia provides a warning sign for potential hemorrhage. Notably, in patients exhibiting dyspnea with diffuse pulmonary infiltrates on chest radiography, decreasing hemoglobin measurements can significantly aid in differentiating between diffuse alveolar hemorrhage and cardiogenic pulmonary congestion.
Our study also has certain limitations. Firstly, the FAERS system is a voluntary reporting system for clinical AEs by physicians, pharmacists, patients, and others, which may introduce reporting bias and omissions of critical information. Notably, most of the PTs related to bleeding were not classified, and many bleeding reports did not record the clinical outcomes of patients, preventing us from categorizing the types of bleeding induced by tirofiban and assessing the severity of bleeding. Additionally, missing data on tirofiban dosing, concomitant antiplatelet/anticoagulant regimens, and key demographic variables limited our ability to evaluate potential confounding factors. Secondly, the disproportionality analysis primarily focuses on the signal strength of AEs, i.e., the correlation between AEs and drug use, rather than establishing a definitive causal relationship. Therefore, our analysis may have conflated adverse reactions with indications, leading to the inclusion of many PTs that describes the cardiac thrombus status of ACS, the secondary cardiac dysfunction, and postoperative complications of PCI, etc. These results made “cardiac disorders” the highest-ranked SOC term, yet excluding these results from the discussion might obscure some potential cardiovascular system-related adverse reactions of tirofiban.
In conclusion, our study, through data collection, screening, and disproportionality analysis of the FAERS database, provides insights into the real-world safety profile of tirofiban. As an antiplatelet agent, bleeding is its most common and clinically significant adverse reaction, especially in the treatment of cerebrovascular diseases considering the possibility of hemorrhagic transformation. Our results not only encompassed all bleeding reactions mentioned in the prescribing information but also report two severe bleeding types: hemothorax and hemorrhagic shock. Additionally, potential adverse reactions not mentioned on the drug label were detected, among which acute respiratory failure had a high frequency and signal strength and should be given attention in clinical practice.