Astronomers hoping to observe a planet around a nearby star have witnessed a much rarer “unprecedented celestial event,” the team said: The violent aftermath of not one, but two collisions between the rocky building blocks of planets.
Over the…
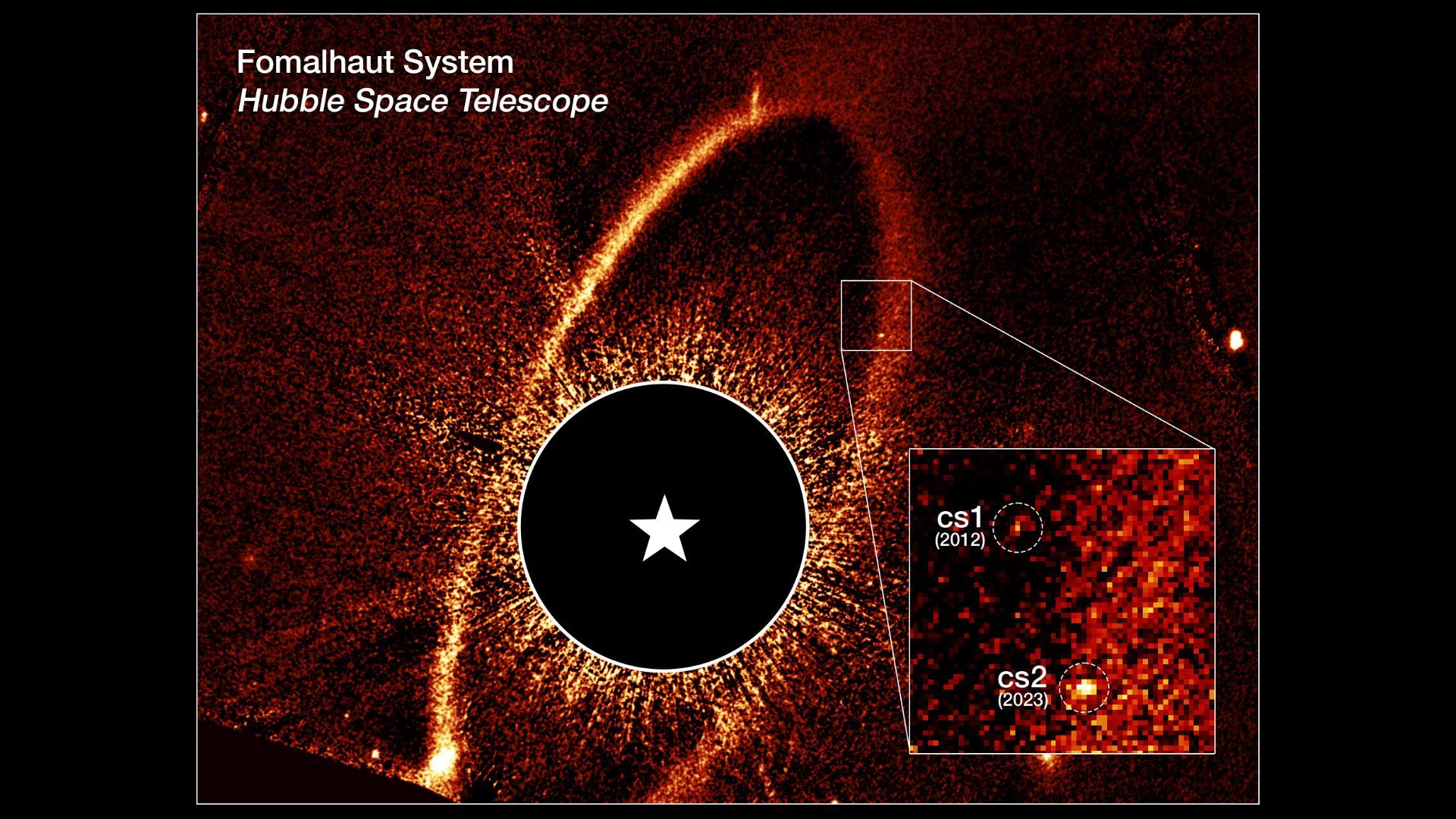
Astronomers hoping to observe a planet around a nearby star have witnessed a much rarer “unprecedented celestial event,” the team said: The violent aftermath of not one, but two collisions between the rocky building blocks of planets.
Over the…