In this study, drug-induced (34.4%) and HBV infection (26.2%) emerged as the predominant factors in patients with ALF, while HBV infection (63.6%) and alcohol-associated factors (16.4%) were identified as key contributors in patients with ACLF. These findings align with prior investigations.
In China, HBV remains a significant nationwide concern, affecting nearly 100 million individuals [16]. HBV is the primary pathogen leading to ALF in mainland China and much of East Asia, while hepatitis E virus is endemic in India and Southeast Asian nations, causing self-limiting illness with a notable risk of ALF in pregnant women. The epidemiology of LF has undergone rapid changes over the past decade. However, recent data indicate a rising incidence of drug and herb-induced ALF across many countries.The frequency of ALF due to drug-induced liver injury (DILI) and other causes is escalating. DILI constitutes a major component of ALF in Western nations, whereas infectious, particularly viral, causes predominate elsewhere [17]. Unlike the Western context, ALF resulting from paracetamol is uncommon, with herbs and traditional medicines being the most frequently implicated agents in China [18]. Severe alcohol-related hepatitis ranks as the second common precipitating factor of ACLF in Western countries, accounting for 25%~40% of cases [2].
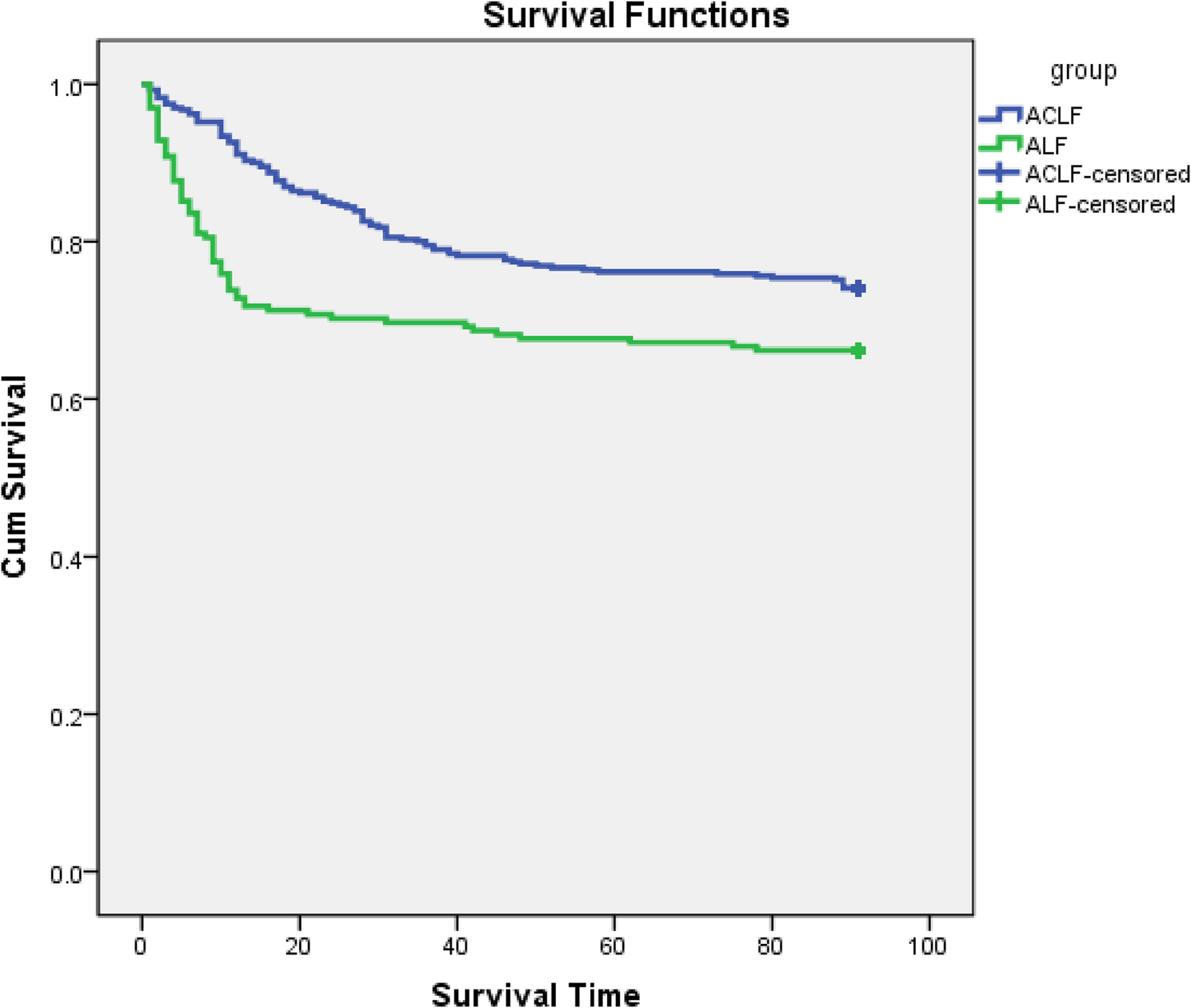
Although numerous studies have investigated ALF and ACLF, there remains a paucity of research comparing their respective survival rates. Our study revealed that ALF patients experienced significantly higher in-hospital mortality 28-day mortality, compared to ACLF patients.
By analyzing previous literature on the definitions and critical illness scores associated with these conditions, we aimed to elucidate the underlying reasons. Prior studies have reported a hospital mortality rate of 40% for ALF patients [19], with 28-day and 90-day mortality rates of 33% and 51%, respectively [20]. In an Australian cohort, 28-day and 90-day liver transplant-free mortality rates were 22.6% and 55.0%, respectively [21].
ALF is often associated with multi-organ dysfunction, attributed to an overwhelming pro-inflammatory state resulting from massive hepatocellular damage and concurrent inflammatory response. Our analysis suggests that the severity of hepatocellular damage and pro-inflammatory response places ALF patients at a higher risk. As a result,ALF patients exhibited higher median critical illness scores compared to ACLF patients. This indicates a more severe clinical presentation and poorer prognosis among ALF patients. Additionally, we observed a higher incidence of MODS and greater number of organ failures among patients with ALF compared to those with ACLF, with percentages of 61% and 45.1%, respectively (p < 0.001). Patients diagnosed with MODS necessitated organ support interventions such as CRRT, MV. This observation suggests a potential association between MODS and poor prognosis, likely due to the increased severity of organ dysfunction.Specifically, patients experiencing persistent failure of three organs face a substantial risk of death within 28 days (70ཞ100%) and 90 days (80ཞ100%) [22].
Early recognition and referral of high-risk patients to specialized centers, coupled with prompt management of liver failure and its complications, may improve patient outcomes [23, 24].
HE manifests as brain dysfunction resulting from liver insufficiency or portosystemic shunting. Patients with advanced HE exhibit a 35% mortality rate in the ICU [25]. Elevated NH3 levels, a known contributor to HE pathogenesis.The results of this study are consistent with those of previous studies, and NH3 were higher in ALF patients compared to ACLF patients (43 vs. 33 µmol/L, P < 0.001).
AKI affects up to 50% of hospitalized patients with liver failure and serves as a strong predictor of poor short and long term survival [26, 27]. Additionally, AKI increases the risk of respiratory failure due to pulmonary edema in patients exhibiting clinical signs of intravascular volume overload.
The proportion of patients with ALF and ACLF diagnosed with septic shock was 17.9% and 16.2%, respectively. Patients with septic shock have an increased ICU and in-hospital mortality [28]. Patients with septic shock exhibit a hyperdynamic circulation with decreased systemic vascular resistance, manifested by low arterial blood pressure and increased cardiac output. LF is potentially reversible, and with early attenuation of the acute precipitating event, liver reserve improves, fibrosis regresses, and portal pressure decreases [29]. Early recognition of LF before the onset of sepsis and extrahepatic insults (such as renal, circulatory, and respiratory failure) is crucial to prioritize organ-specific interventions [30].
Mortality rates among patients requiring MV can be as high as 49.6%, highlighting its association with poor prognosis. Moreover, respiratory failure requiring MV was associated with the highest 28-day mortality rate (83.7%) in LF patients [20].
In this study, we found that the proportion of ALF and ACLF patients diagnosed with sepsis was 80.5% and 77.9%, respectively, indicating a high occurrence of sepsis in LF patients.
LF is characterized by immune dysfunction and dysregulated immune cell activation, leading to an increased risk of bacterial infections in patients with liver failure [31].Immune dysfunction is central to LF pathogenesis and is believed to contribute to its infectious complications and their adverse effects on patient survival [32].These dysfunction ultimately lead to acquired immunodeficiency, impairing the host’s antimicrobial responses and increasing susceptibility to infections [33, 34]. Monocytes and macrophages play a crucial role in disease pathogenesis in both ALF and ACLF, driving local inflammation, tissue repair, and systemic complications [19].
Sepsis can precipitate the progression of LF [35, 36]. The mortality rates of patients with cirrhosis requiring ICU admission for sepsis and septic shock was ranging from 60–76% [37]. These patients also face a high risk (40%) of hospital infection with both bacterial and fungal pathogens, with up to a four-fold increase in mortality [38]. Bacterial infections are among the most common triggers of LF, with reported rates of bacterial and fungal infections of up to 80% and 32%, respectively, in patients with ALF [39]. SBP and pneumonia are the top two common infectious complications, often caused by multidrug-resistant organisms [40].
The NLR serves as an indicator of systemic inflammation based on complete blood count values.Patients with ALF exhibited a higher median admission leucocyte count and a relatively higher median admission NLR. These levels were significantly higher than the normal values, indicating severe systemic inflammation.Multivariate logistic analysis in ACLF patients revealed that a higher NLR (OR = 1.075, 95% CI 1.027–1.126, P < 0.001) was associated with increased 90-day mortality.
Neutrophil count experiences a substantial increase, followed by migration of these neutrophils to affected areas.Generally, neutrophil count in blood increases with the progression of inflammatory diseases.Consequently, lymphocyte count decreases due to immunosuppression. Moreover, the release of various anti-inflammatory cytokines into the bloodstream induces immunosuppression, leading to apoptosis of numerous lymphocytes [41, 42].
NLR increased with disease progression, particularly in inflammatory diseases, and this increase correlated with the development of certain diseases. These findings suggest that higher NLR values are independently associated with unfavorable clinical prognosis in patients with sepsis [43]. Recent evidence supports the utilization of NLR as a biomarker for predicting 90-day mortality risk in ACLF patients [44, 45].
Despite being considered a promising and cost-efficient method for predicting mortality in critically ill patients with cirrhosis, there is ongoing debate regarding the most accurate cutoff value associated with high risk of poor outcomes. One study indicated that the mean normal NLR for men and women were 1.63 and 1.66, respectively [46]. Another study demonstrated that NLR ≥ 2.72 in stable outpatients with cirrhosis was linked to increased mortality [47].
However, in some conditions such as cachexia, the count of neutrophil may not increase. As inflammation progresses, the lymphocyte count decreases. However, the decrease is relatively delayed and may not accurately reflect disease progression [42]. These factors may explain why NLR did not significantly affect the 90-day mortality of ALF.
LF is a dynamic, multisystem process characterized by multiple defects and abnormalities in both cellular and soluble components of the immune system. These defects ultimately result in acquired immunodeficiency, which impairs the host’s antimicrobial responses and increases susceptibility to infections [33, 34].Immune dysfunction is central to the pathogenesis of LF and is hypothesized to contribute to infectious complications and their adverse effects on patient survival [31, 32, 48].
In this study, 78.8% of LF patients were diagnosed with sepsis, indicating a relatively high prevalence.
Given the severe organ dysfunction and immunocompromised state in patients with LF, they are particularly vulnerable to sepsis, which can exacerbate organ failure [49]. Any patient with confirmed infection should undergo a thorough evaluation for sepsis or septic shock, as well as for potential organ dysfunction.
The Sepsis-3 criteria should be employed respectively to assess these conditions. These evaluations are essential for identifying patients at increased risk of mortality who may require more intensive care interventions [50].
Consequently, early identification of LF and sepsis prior to the development of sepsis and extrahepatic insults, such as renal, circulatory, and respiratory failure, is crucial for prioritizing organ-specific interventions.
Our study had several limitations. Firstly, it is crucial to acknowledge that this study was conducted as a single-center investigation, which may restrict its generalizability to other settings. Secondly, retrospective studies are susceptible to selection bias. Thirdly, single-center studies often face challenges in adequately controlling for all potential confounding factors that may influence the outcomes.A limitation of this study is its longitudinal design spanning 13 years. Over this extended period, the implementation of different treatment protocols likely introduced confounding variables, thereby affecting the final outcomes to some degree.