Bioinspired liquid crystal actuators unlock programmable climbing and shape-shifting motion
Bioinspired liquid crystal actuators unlock programmable climbing and shape-shifting motion
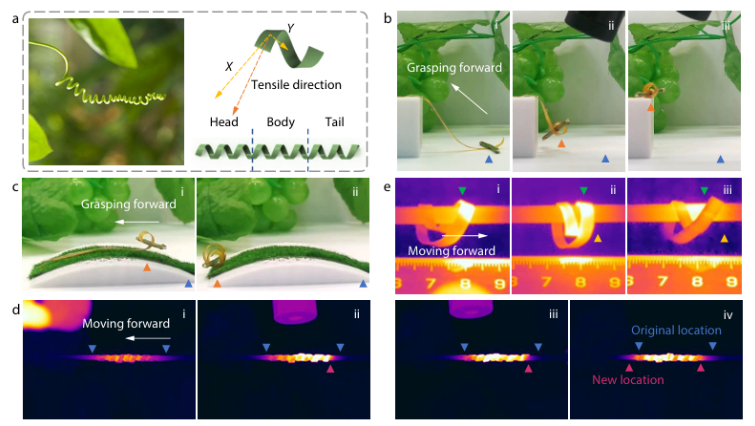
KNOXVILLE, TN, December 24, 2025…
Bioinspired liquid crystal actuators unlock programmable climbing and shape-shifting motion
Bioinspired liquid crystal actuators unlock programmable climbing and shape-shifting motion
KNOXVILLE, TN, December 24, 2025…