Sept. 10, 2025 — There’s an important component to brain health in our heads – a complex, tightly packed barrier that surrounds the brain called the blood-brain barrier (BBB). This powerful protector makes sure nothing harmful, such as toxins, gets into our brains. But there’s a downside – it also makes it hard to deliver much-needed medicines to their targets in the brain.
Currently, 98% of drug candidates in early phases of the drug industry cannot cross the BBB, creating a significant challenge for the development of treatments for neurological (Alzheimer’s and Parkinson’s diseases), psychiatric (depression, anxiety) and brain cancer diseases. Think of the BBB as a bouncer at a popular nightclub. The bouncer lets in necessary nutrients, like Omega-3 fatty acids, which are essential for brain development and health, because they’re dressed just right. But the brain club is highly exclusive, and the bouncer sees almost everything else as a problem partier, even medicine that could be life-saving.
Researchers from the Weill Medical College of Cornell University (Weill Cornell Medicine) are working to discover what happens at the BBB and how that bouncer chooses what to let in and what to deny entry. They’ve been able to make great strides in their work due to the powerful resource, Delta, at the National Center for Supercomputing Applications (NCSA).

The bouncer analogy is how Margarida Rosa, a doctoral candidate at Weill Cornell Medicine, describes the process of moving molecules past the BBB to laymen to help them understand it better. She’s working with George Khelashvili, Ph.D, an associate professor at the Department of Systems and Computational Biomedicine at Weill Cornell. In the lab, the research team investigates a special protein that acts like a kind of VIP pass through the usually restrictive BBB. This protein is called MFSD2A (Major Facilitator Superfamily Domain 2A). It’s found in the cells lining the blood vessels of the brain within the BBB, and, importantly, it’s what escorts Omega-3 through the barrier. This protein system is of high interest to the team at Weill because if they can figure out how MFSD2A escorts Omega-3, they could apply that knowledge to make it escort specific drug-like molecules past the BBB, making many medicinal treatments much more effective.
This is where MFSD2A could be the key to getting medicine directly to the brain. Research has shown that MFSD2A can transport more than Omega-3s through the BBB – certain drugs, such as tunicamycin antibiotic, could also be carried through – but the protein won’t carry just any molecule. Rosa and Khelashvili are working to uncover the specific qualities a molecule must possess to be carried by MFSD2A, with the goal of designing small therapeutic drugs that can pass safety through the BBB.
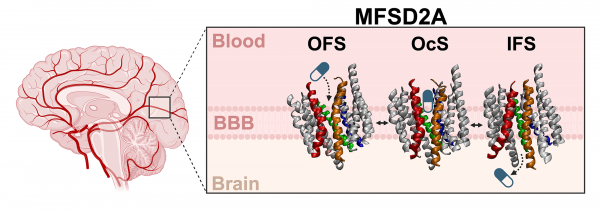
To perform this research, the team utilized research computing to create highly detailed simulations that allowed them to study how MFSD2A operates at an atomic level.
“I use computer simulations to get a close-up view of how this protein moves molecules into our brain and keeps others out,” said Rosa. “My aim is to find out the important features that make a molecule ‘party-ready’ for the brain. Just like wearing the right outfit might get you into a party, certain features of a molecule, such as size, charge or hydrophobicity, can make it more likely to be transported by MFSD2A.”
By analyzing these long simulations with advanced computational biophysics and machine learning approaches, the team can train computer models to recognize the differences between molecules that MFSD2A will transport and those it won’t. “Once I figure out these differences,” explained Rosa, “I can then modify the molecules that usually cannot enter the brain by adding features that will make them more likely to be transported. I will ‘dress them up’ in a way that makes MFSD2A want to let them in. Then I can test whether these ‘outfits’ persuade the bouncer (BBB) to let these molecules enter the brain through MFSD2A.”
These simulations have given the research team a lot of insight into how MFSD2A operates, including which transitions, steps and specific residues are important for transporting Omega-3 through the protein. They used an advanced mathematical framework called a Markov State Model (MSM), together with specialized machine learning (ML) approaches, to track how the protein conformation changes over time and identify the key steps in MFSD2A-mediated transport of nutrients through the BBB. In their published work, these simulations also helped explain how mutations can disrupt MFSD2A’s function. Specifically, MFSD2A’s dysfunction has been linked to serious brain conditions like Alzheimer’s disease, microcephaly (a condition where the brain doesn’t grow properly), brain damage from sepsis and brain bleeding.
Once the simulation results are analyzed, the team collaborates with experimental researchers at Columbia University and the University of Queensland to test their predictions. “Integrating computational findings with experiment makes this a truly multidisciplinary research strategy, allowing the formulation of mechanistic hypotheses that can be directly tested. This approach deepens our understanding of MFSD2A’s function and lays the groundwork for rational drug discovery targeting MFSD2A during its dysfunction and the blood-brain barrier, in general,” said Rosa.
More recently, Khelashvili and Rosa have been running simulations to compare a normal functioning protein to one that’s impaired, and in doing so, they’ve discovered that certain ions can block the MFSD2A from transporting effectively by binding to the protein in a specific way. The team is now preparing a paper that builds on these findings, focusing on how lithium – a common treatment for bipolar disorder – inhibits the function of MFSD2A and the molecular mechanism behind this effect. While lithium remains an important therapy, its side effect on MFSD2A may represent an unintended off-target effect. The group also continues to use their methodology to study MFSD2A mutants that alter the transport of Omega-3 across the BBB, including both those that enhance and impair the protein’s ability.

Khelashvili and Rosa believe that their project will have an impact in a number of areas. Not only are they optimistic that this research will help with the development of drugs that can be delivered directly to the brain via MFSD2A, but they also hope their research into how MFSD2A functions will help other teams research similar lipid transporters.
“MFSD2A belongs to a small subset of atypical MFS transporters, which, unlike most other members of the superfamily, transport amphipathic lipidic substrates rather than water-soluble molecules. This gave rise to the hypothesis that the lipid transporter members of the superfamily likely function according to a common mechanism that could differ from that proposed – and extensively studied – for the MFS solute carriers. Our project constitutes a unique opportunity to tackle this problem,” said Khelashvili.
One of the more impactful aspects of this study is that it provides a strong example to inspire other researchers to use a similar approach. “The use of advanced computational methods, such as molecular dynamics simulations, MSMs and ML analyses, combined with validation through biochemical (e.g., single-cell transport assays and scintillation proximity assays) and biophysical (e.g., CryoEM) experiments, represents the forefront of drug discovery technology,” said Rosa. “This highly integrative approach promises to streamline the drug discovery process, offering innovative platforms and insights.”
“This research supports drug discovery by learning how MFSD2A transports specific molecules, and we aim to create drugs that leverage this mechanism to cross into the brain, potentially improving treatment options for neurological conditions such as Alzheimer’s disease, depression and brain cancer,” said Khelashvili.
Much of this work would not have been possible without the resources that NCSA can provide. Khelashvili and Rosa used the U.S. National Science Foundation’s ACCESS program to secure time on the Center’s GPU-based supercomputer, Delta.
“MD simulations are used to model the behavior of atoms and molecules over time, giving very detailed and important information you cannot obtain from experiments. But biologically meaningful events often occur on the microsecond to millisecond scale, well beyond the reach of standard simulation techniques,” said Rosa. “MFSD2A is also a large and complex system (approximately 230,000 atoms when embedded in the membrane and immersed into a solution environment) with rare transport events, requiring access to the latest GPUs, parallel computing infrastructure and enhanced sampling techniques, all available through ACCESS.”
“Using HPC resources, we achieved sampling on unprecedented time-scales, over 3 milliseconds of cumulative MD trajectories across different MFSD2A constructs, including various conditions and mutants. Analyses of this massive MD data allowed us to extract detailed and statistically robust information about molecular interactions, inhibitory transport-enabling and transport-inhibiting conditions – insights that are critical for understanding the transport mechanism and for informing drug discovery. These simulations were completed in a matter of months using parallel scheduling via Slurm ensemble jobs, which would have taken years on a standard workstation.”
For those interested in more detailed information about this research, in addition to the forthcoming publication, there are several articles of note that the team has published on this work:
Structural Basis of Omega-3 Fatty Acid Transport across the Blood–Brain Barrier, Nature
Substrate Binding-Induced Conformational Transitions in the Omega-3 Fatty Acid Transporter MFSD2A, Nature Communications
Automated Collective Variable Discovery for MFSD2A Transporter from Molecular Dynamics Simulations, Biophysical Journal
Source: Megan Meave Johnson, NCSA
