Scientists at Merck & Co. have unveiled the structure of the company’s latest candidate for HIV pre-exposure prophylaxis (PrEP). The compound, known as MK-8527, is designed to be taken as a pill once a month to prevent HIV infection and is currently in Phase 3 clinical trials for HIV PrEP (PLoS Biol. 2025, DOI: 10.1371/journal.pbio.3003308).
HIV-PrEP drugs currently on the market are either pills that need to be taken daily, such as Truvada (emtricitabine and tenofovir disoproxil fumarate) and Descovy (emtricitabine and tenofovir alafenamide fumarate), or long-acting injections, such as Apretude (cabotegravir) and Yeztugo (lenacapavir), which are given every 2 or 6 months, respectively.
The key for drugmakers is to give people options that ensure they adhere to HIV-PrEP drug regimens, says Merck chemist Izzat T. Raheem, who led the development of MK-8527 along with his colleague Tracy L. Diamond. “That’s why these longer-acting regimens, we believe, have a huge role to play,” he says. Oral medications are accessible, convenient, and discreet.
MK-8527’s development grew out of the pharmaceutical company’s islatravir program. Islatravir is currently in clinical trials as a treatment for HIV when used in combination with the approved drug Pifeltro (doravirine). Both islatravir and MK-8527 are nucleoside reverse transcriptase translocation inhibitors (NRTTIs)—a new class of antivirals that use a couple of mechanisms to muck up HIV’s ability to replicate. When incorporated into a growing DNA strand, NRTTIs prevent translocation, the process in which HIV’s reverse transcriptase enzyme moves to the next site on the template DNA. In the event that translocation still occurs, NRTTIs introduce a conformational change in the DNA that stops the chain from continuing to grow.
Raheem tells C&EN that the scientists explored hundreds of molecules in the process of going from islatravir to MK-8527. Ultimately, there are two key differences between the two molecules: a nitrogen in islatravir’s nucleobase is replaced by a carbon in MK-8527, and a fluorine is swapped for a chlorine.
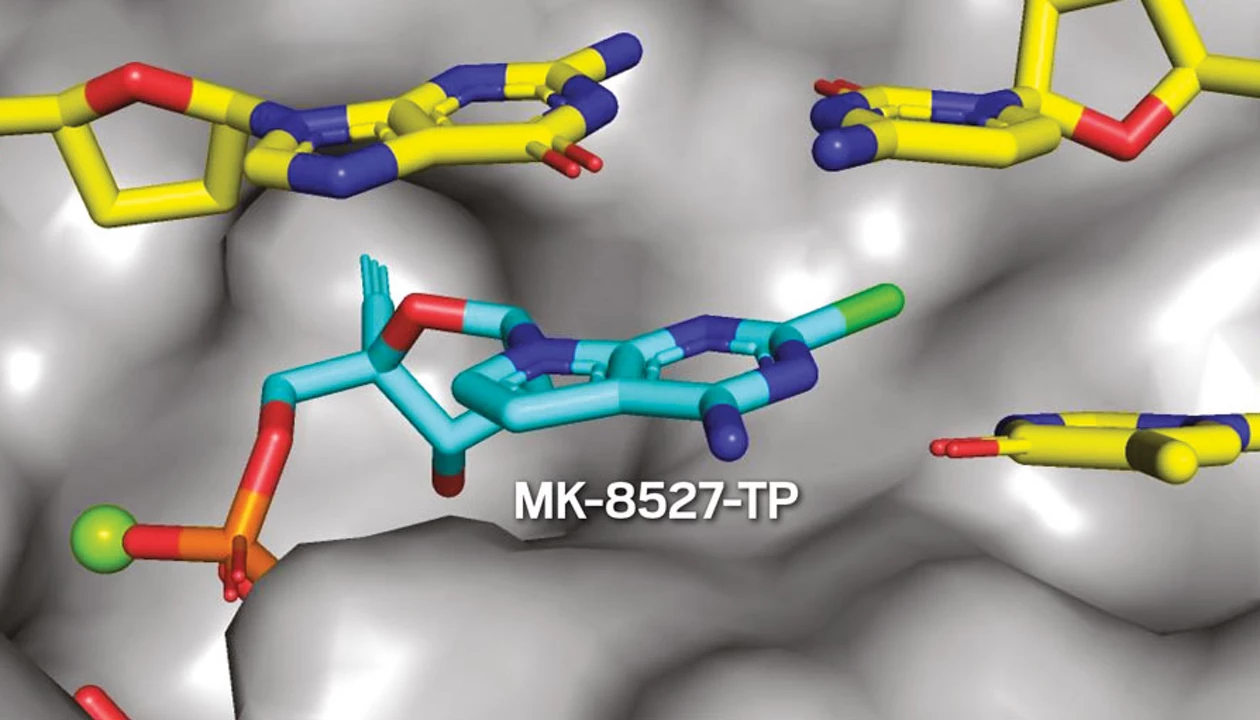
Raheem points out that both molecules have an alkyne group that is critical to their success. “That alkyne sits in a really important pocket, and we believe it is part of the actual physical driver for preventing translocation,” he says. Attempts to swap the alkyne for similar substituents, such as a nitrile, weren’t successful.
The alkyne also caught the eye of Dennis Liotta, a professor at Emory University who has extensive experience developing HIV therapeutics. He points out that an X-ray crystal structure in the Merck group’s publication reveals that “there’s a little pocket where that alkyne fits, and just about nothing else fits exactly as snugly.”
Liotta also notes that there’s a need for HIV PrEP that’s dosed more frequently than lenacapavir. “I think there are legitimate concerns about having something in your body for 6 months that has at least a fair number of drug-drug interactions,” he says. MK-8527’s monthly dosing regimen could be in a sweet spot that helps people be compliant with the regimen but also sidesteps the problem of contraindicated medications, should the need to take one arise.
Chemical & Engineering News
ISSN 0009-2347
Copyright ©
2025 American Chemical Society