RIYADH: The Royal Commission for AlUla announced on Thursday the discovery of rare horseshoe crab fossils estimated to be 465 million years old.
Work is being carried out to study and characterize the fossils, which have not yet been…
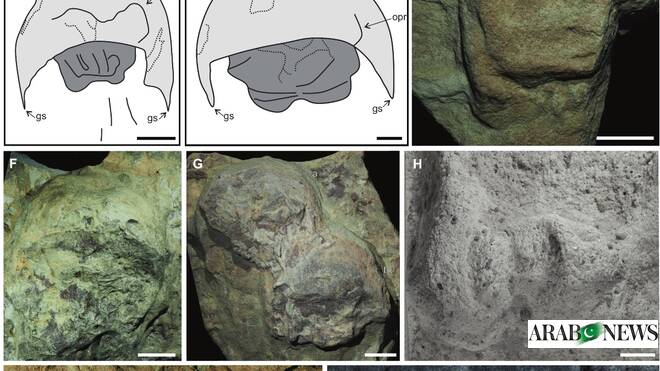
RIYADH: The Royal Commission for AlUla announced on Thursday the discovery of rare horseshoe crab fossils estimated to be 465 million years old.
Work is being carried out to study and characterize the fossils, which have not yet been…