China’s first Mars rover, Zhurong, has provided groundbreaking insights into the history of water on Mars, showing that liquid water existed on the planet much later than scientists previously believed. According to a recent study…
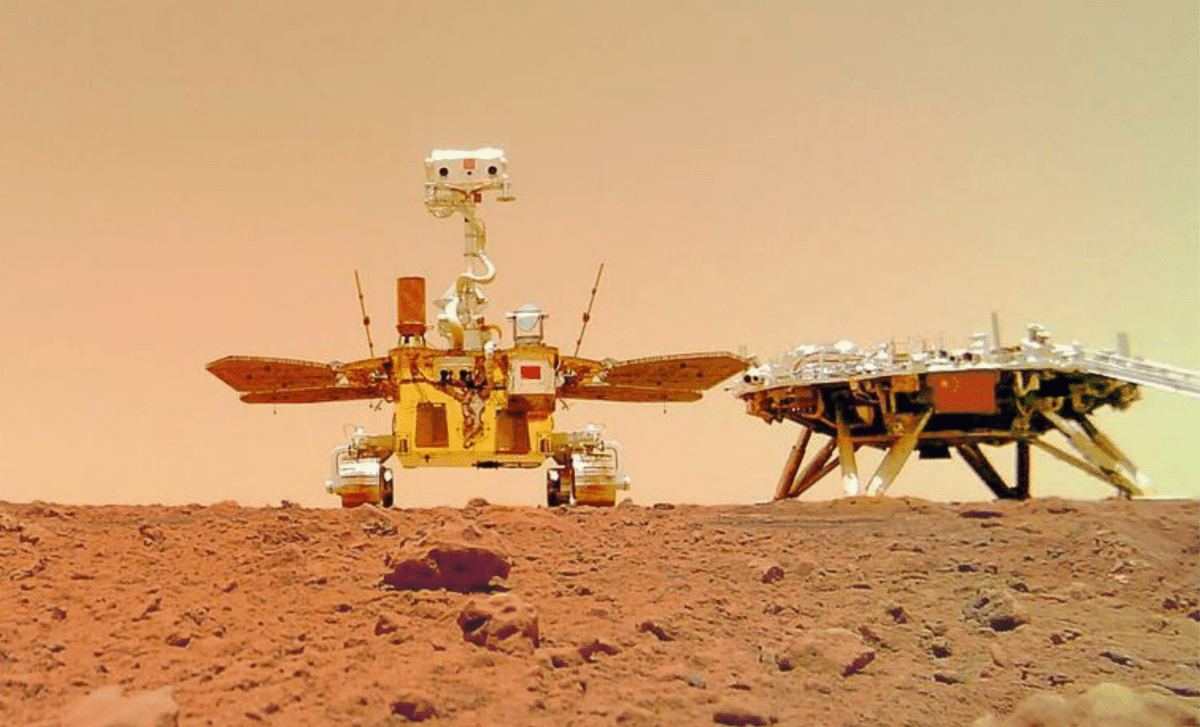
China’s first Mars rover, Zhurong, has provided groundbreaking insights into the history of water on Mars, showing that liquid water existed on the planet much later than scientists previously believed. According to a recent study…