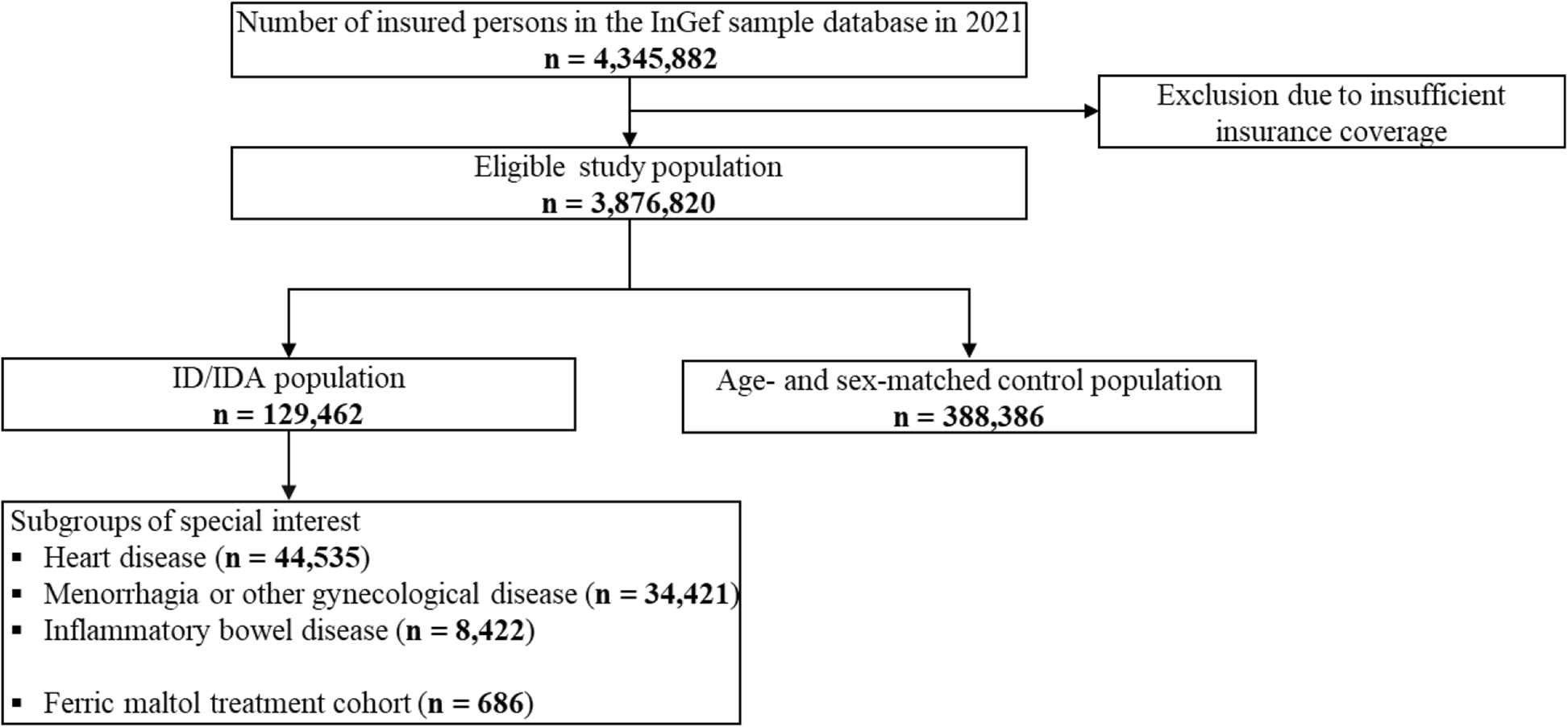
Data selection and patient flow
For each year of the observational period (2016–2021), study populations were identified, as shown for 2021 (Fig. 1).
Flow of individuals through the study, exemplarily shown for 2021. Individuals were identified for each year of the study period (2016, 2017, 2018, 2019, 2020, 2021) to define study populations, except ferric maltol treated patients (treatment cohort; index period 2017–2020; ID/IDA: iron deficiency/iron deficiency anemia)
In 2021, 129,462 individuals were diagnosed with ID and/or IDA, and subgroups of special interest were formed on the basis of additional criteria (heart disease, geriatric, menorrhagia or other gynecological diseases, gastrointestinal bleeding, inflammatory bowel disease) or ferric maltol treatment (number of individuals during the study period: see Additional file 1, Supplementary Tables 2–3).
Prevalence
The resulting one-year prevalences of ID/IDA obtained from the database and extrapolated to the German population were stable over the study period at approximately 3.3% and 3.2%, respectively, corresponding to approximately 2.7 million individuals affected in Germany each year (Table 1).
As shown for 2021, overall prevalence was higher in individuals with advanced age (< 18 years: 0.84% [95% CI, 0.82–0.86], >90 years: 9.87% [95% CI, 9.6–10.16]). Women were more affected than men, in particular in their reproductive age, with prevalences of 5.60%, 6.86%, and 7.35% in the age cohort of 18–29 years, 30–39 years, and 40–49 years, respectively (Fig. 2).
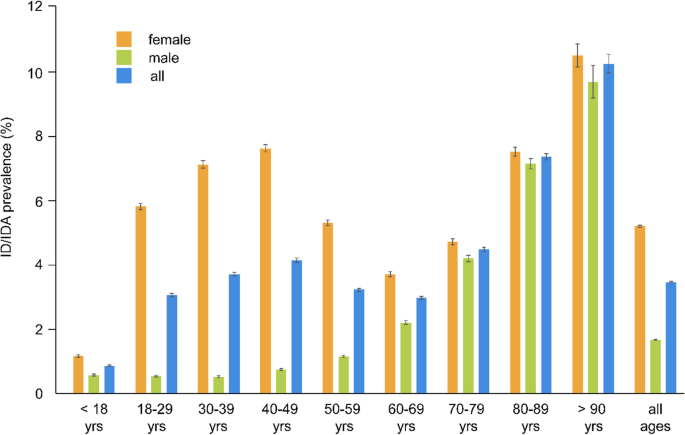
Prevalence of ID/IDA with 95% confidence interval in Germany, 2021 (ID/IDA: iron deficiency/iron deficiency anemia; yrs: years)
Comorbidity burden
In 2021, 32.1% of patients with ID/IDA and 11.5% of matched control individuals had six or more diseases of the ECI. When compared to control individuals, this comorbidity burden was further increased in patients with ID/IDA with concurrent menorrhagia or other gynecological diseases (17.9%), inflammatory bowel disease (37.1%), and heart disease (72.6%; Fig. 3).
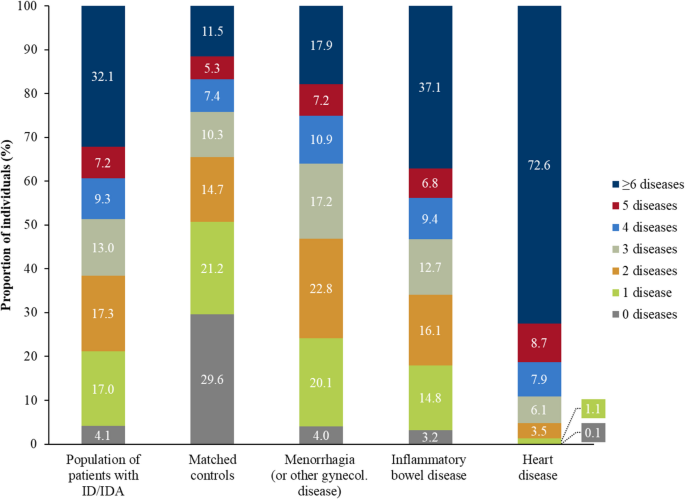
Comorbidity burden of patients with ID/IDA, matched control individuals, and patients with ID/IDA with concurrent diseases in Germany, 2021 (ID/IDA: iron deficiency/iron deficiency anemia)
During each year of the study period, the mean number of diseases of the ECI of the overall population of patients with ID/IDA was two-fold higher than that of matched control individuals (mean ± SD: 4.5 ± 3.6 vs. 2.3 ± 2.5 in 2021) and was higher among subgroups (mean ± SD: 3.4 ± 2.8 [menorrhagia or other gynecological diseases], 5.0 ± 3.9 [inflammatory bowel disease], and 7.9 ± 3.5 [heart disease]).
The most commonly observed pre-defined diagnoses in patients with ID/IDA with concurrent menorrhagia or other gynecological diseases were menopausal and other perimenopausal disorders (42.0%) and excessive, frequent and irregular menstruation (41.6%), while the most frequently observed pre-defined diagnoses in patients with ID/IDA with concurrent inflammatory bowel disease were noninfective gastroenteritis and colitis (61.1%), colitis ulcerosa (27.0%), and Crohn’s disease (25.7%). In patients with ID/IDA with concurrent heart disease, the most commonly observed pre-defined diagnoses were chronic ischemic heart disease (47.1%), heart failure (45.8%), and type 2 diabetes mellitus (40.6%) (Table 2).
Mean number of diseases of the ECI ± SD throughout the study period as well as additional data on further subgroups are provided in Additional file 1, Supplementary Tables 4–5).
Treatment of iron deficiency and iron deficiency anemia
Between 2016 and 2021, most of the patients with ID/IDA who were prescribed iron preparations received at least one dispensation for an oral bivalent iron preparation (25.0% – 27.5%), followed by parenteral (5.8% – 6.5%) and oral trivalent iron preparations (0.2% – 0.7%; (Fig. 4A).
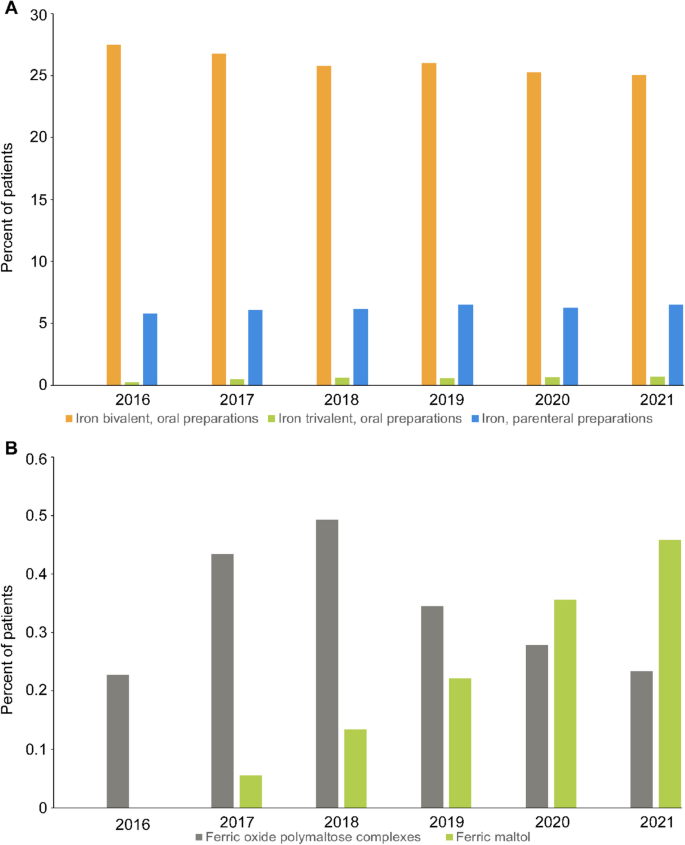
Usage of supplemental iron formulations in Germany, 2016–2021. A Percentage of patients with ID/IDA receiving oral bivalent, oral trivalent, and parenteral iron preparations during the study period. B Percentage of patients with ID/IDA receiving the oral trivalent the preparations ferric oxide polymaltose complex and ferric maltol (ID/IDA: iron deficiency/iron deficiency anemia)
Iron (Fe2+)-glycine sulfate and iron (Fe2+)-sulfate were the most widely used medications within the class of oral bivalent iron formulations, and ferric oxide polymaltose and ferric sodium gluconate complexes dominated the class of parenteral formulations. The ratio of medications within each class of formulations was almost unchanged over the study period (Table 3).
For the rarely prescribed oral trivalent iron preparations, however, the use of ferric maltol increased slightly between 2018 and 2021 when compared to ferric oxide polymaltose complexes (Fig. 4B). Data on other subpopulations of patients with ID/IDA are provided in Additional file 1, Supplementary Tables 6–10.
Healthcare resource utilization and expenditures
In the population of patients with ID/IDA, the percentages of both hospitalized individuals (all-cause) and individuals on sick leave were higher than in the matched control group (41.2% vs. 22.0%; 21.4% vs. 3.1%). These differences corresponded to higher mean total costs (all-cause) in patients with ID/IDA than in the matched control group (Table 4).
ID/IDA-related costs (mean ± SD) for patients with ID/IDA (472 ± 2,303 €) further increased with concurrent diseases and were highest for patients with concurrent heart disease (853 ± 3,535 €). HRU and associated expenditures in subgroups are depicted in Additional file 1, Supplementary Tables 11–12.
Ferric maltol treatment
One year after treatment initiation with ferric maltol, HRU of the treatment cohort was lower in important parameters when compared to the one-year period before the index date (Table 5).
These parameters included the percentage of patients with hospitalizations due to ID/IDA (1.5% vs. 3.1%), the percentage of patients using outpatient services due to ID/IDA (68.8% vs. 96.4%), and the percentage of patients with at least one sick leave due to ID/IDA (0.7% vs. 1.9%). These results were consistent among all subgroups for HRU (see Additional file 1, Supplementary Tables 13, 15, 17, 19, and 21) and associated costs (see Additional file 1, Supplementary Tables 14, 16, 18, 20, and 22). The total ID/IDA-related mean costs were higher after treatment initiation when compared to the pre-index period (609 ± 1,674 € vs. 358 ± 597 €), potentially driven by higher costs for drug treatment. However, all-cause costs remained approximately the same from the pre-index to the post-index period (11,866 ± 18,170 € vs. 11,866 ± 15,840 €; Table 5). It is interesting to note that the total mean treatment costs of this cohort were considerably higher than in the whole population of patients with ID/IDA, even before treatment initiation with ferric maltol (Tables 4 and 5).
Within the first 180 days after treatment initiation with ferric maltol, the majority of patients with ID/IDA and patients with ID/IDA with concurrent disease did not switch to any other iron preparation (Table 6).
The post- vs. pre-index comparison revealed that ferric maltol treatment was associated with a considerable 29.0% point decrease in the proportion of patients receiving no treatment (from 64.0% before to 35.0% post index date; Table 7).
Among patients who received iron preparations, the proportion of those with parenteral iron preparations decreased (from 52.6% before to 37.4% post index date; Table 7). In the subgroups analyzed, the decrease in the proportion of patients receiving no treatment was between 26.5 (menorrhagia and other gynecological diseases; from 63.7% before to 37.2% post index date) to 33.3 (heart disease; from 63.6% before to 30.3% post index date) percentage points (see Additional file 1, Supplementary Tables 23–27).