Varroa-bee system
Western honeybees (Apis mellifera) are vital agricultural pollinators, contributing millions of dollars annually to the global economy [49]. Despite their pivotal role in food production, honeybee colonies have faced significant declines in recent decades [50,51,52]. One major driver of these losses is the infestation by Varroa destructor mites, specialised ectoparasites that feed on fat body and haemolymph of honeybees, and vector of various pathogenic microorganisms, severely compromising honeybee physiology [53].
Considering holobionts as structurally and functionally dynamic units implies that their taxonomic composition and microbial assemblage are subject to variability influenced by biotic and abiotic ecological determinants. However, like any system, they must maintain a certain level of stability, which is why they are characterised by the existence of a conserved microbial core, generally resistant to external perturbations. Corresponding to this, the honeybee gut microbiota has been show to consist of core bacterial taxa, including Lactobacillus (Firm-4 and Firm-5), Bifidobacterium, Snodgrassella alvi, Gilliamella apicola and Frischella perrara, as well as Bartonella apis, Bombella apis and Commensalibacter [12]. These bacteria play a nutritive role, promote detoxification of dietary compounds and pesticides and provide protection against pathogens by inhibiting harmful microbes, such as Paenibacillus larvae [13], through metabolic and immune-supportive functions [14,15,16]. The successful completion of the core’s essential functions within the system demonstrates a successful process of microbial interaction. From this, one can hypothesise that, while ecological competition may exist owing to exploitation or interference, what prevails within the microbial core of the holobiont are synergistic and mutualistic interactions designed to maintain systemic stability. Overall, the absence of antagonistic relationships within the core suggest a stable ecological configuration with a prevalence of potentially mutualistic and functionally complementary interactions.
Interestingly, these genera can also be found in the microbiome of Varroa mites, which confirms that honeybees and their infesting Varroa mites share common bacterial taxa [54, 55]. The presence of common bacterial genera in the microbiomes of both systems suggests possible microbial transfer during prolonged contact at the permissive honeybees–Varroa mites interface. This phenomenon implies the absence of colonisation resistance and, consequently, the establishment of the transferred microorganisms in the mite and the possible formation of functional microbial cores. Community reconfiguration occurs, leading to ecological stability based on possible mutualistic interactions as cross-feeding or syntrophy. In this context, the holobiont maintains a stable microenvironment that allows for microbial co-evolution (Table 1).
The research conducted by Skičková et al. [54] revealed that the keystone in the microbiomes of honeybees and Varroa mites infected by Paenibacillus spp. closely align with the findings of Moran [12]. In the microbiome of honeybees highly infected with Paenibacillus, the keystone taxon was Bifidobacterium, whereas in mites with low Paenibacillus loads, members of the Orbaceae family were predominant. In honeybees with low Paenibacillus infection, the keystone taxa were Lactobacillus and Gilliamella, while in lowly infected Varroa, it was Snodgrassella [54]. These findings suggest that while Paenibacillus spp. can proliferate on both honeybee and Varroa holobionts, each system provides an ecological environment that shapes microbial interactions differently. Furthermore, the passive nature of bacterial transfer to the mite likely results in a low inoculum density, allowing for greater colonisation resistance and a lower microbial load. As a result, the microbiome in mites remains relatively stable, and the emerging social microbial dynamics differ from those in the honeybee. This differentiation suggests that similar microbial taxa can behave differently depending on specific host microbial architectures and ecological pressures, reflecting a nuanced pattern of holobiont–holobiont interactions.
The study also identified the positive and negative network associations, on the basis of eigenvector centrality, between Paenibacillus and other bacterial genera. In highly infected honeybee networks, Paenibacillus was positively linked with three genera: Enterococcus, Bifidobacterium, and Lactobacilllus, and negatively associated with Spiroplasma. However, in Varroa mites highly infected with Paenibacillus, Paenibacillus was only negatively associated with Pseudomonas [54]. Since various Paenibacillus species produce secondary metabolites (e.g. polymyxins, polyketides and paenilarvins), which possess antimicrobial and antifungal effects [56, 57], it is suggested that these compounds could suppress the growth of certain bacterial species that might form interactions within the microbiome. The production of such bioactive compounds can contribute to maintaining a balanced microbial environment by inhibiting the colonisation of potentially pathogenic or competitive bacteria [58]. The antimicrobial activity of Paenibacillus restructures the community and interferes with the colonisation of microorganisms, but it does so within a localised context in restricted compartments of the holobiont. This concentration of activity contributes to the ecological stability of the system, as microbial taxa operate within defined microenvironments, preventing the destabilisation of functionally specific microbial cores.
Although the interactions between Paenibacillus and the adult honeybee microbiota have not been extensively studied, Truong et al. demonstrated that certain Lactobacillus species inhibit the growth of P. larvae and reduce its harmful effects in honeybee larvae [59]. Honeybee Lactobacillus species and other lactic acid bacteria produce several bioactive compounds that can contribute to maintaining a balanced microbial environment by inhibiting the colonisation of potentially pathogenic or competitive bacteria [60]. Limiting the growth of some bacteria, especially pathogens, helps promote the survival of beneficial microorganisms, thus enhancing host health. In addition, the antimicrobial and probiotic effects of some core bacterial strains have an impact on broader microbial community, influencing the dynamics of microbial populations and their interactions with the host. This ecological role of secondary metabolites may also contribute to shaping the resilience of the microbiome to environmental stressors or pathogen challenges (Fig. 2) [59]. The ecological relevance and keystone taxon status of Lactobacillus in the honeybee microbiome suggest the possibility of passive transfer to Varroa destructor during close contact at the bee–mite interface. Once inside the mite, these bacteria could modulate the microbial composition of Varroa, either transiently or through more stable colonisation, thereby influencing the structure of its microbiome and possibly its vectorial capacity. Such microbial transfer represents a potential mechanism of holobiont-to-holobiont cross-talk, with implications for the evolution of symbiotic relationships and the development of biological control strategies targeting vector-associated microbiomes.
Schematic illustrating the multifaceted interactions between the honeybee holobiont (Apis mellifera) and the mite holobiont (Varroa destructor). Each holobiont consists of its respective host or ectoparasite, and associated gut microbiota, with microbial networks that influence and respond to each other through multiple mechanisms. Bacterial sharing reflects overlapping core microbiota taxa, while pathogen transmission, such as with deformed wing virus (DWV), is facilitated by the mite, contributing to honeybee morbidity. The potential involvement of P. larvae is shown for conceptual completeness; however, current evidence does not confirm direct transmission from Varroa to honeybees. Varroa feeding triggers immune activation in the bee (green arrows), including upregulation of antimicrobial peptides (AMPs) and ribonucleic acid interference (RNAi). The bee’s microbiota also plays a role in immune priming and pathogen suppression. Both Paenibacillus and DWV can induce dysbiosis (dashed arrows), disrupting the microbiome balance within either holobiont. Microbial network effects (indicated as +/−) illustrate complex intra-holobiont microbial interactions. Bee defensive responses (immune- and microbiome-mediated) are hypothesized to impair Varroa fitness and reproduction; however, these effects require further experimental validation. Nevertheless, the predominant outcome remains detrimental to the honeybee and nutritionally beneficial to the mite. The system exemplifies a dynamic, bidirectional holobiont–holobiont interaction, influencing host and parasite fitness, immunity, and disease susceptibility
Varroa mite infestation and associated pathogens trigger extensive transcriptional changes in honeybees [61,62,63]. These changes include upregulation of immune system genes coding for antimicrobial peptides (AMPs), RNA interference (RNAi) machinery, and recognition molecules [61,62,63,64], which can also affect the honeybee gut microbiota. Some studies report a significant increase in gut microbial diversity following Varroa infestation [65], while others found no remarkable differences (Table 1) [66].
Varroa mite has become an efficient vector of several deadly honeybee viruses, including deformed wing virus (DWV), which is one of the most significant threats to A. mellifera honeybees (Table 1). Pathogenicity can be triggered in bees even with naturally low viral loads by some bacterial co-infections. For example, honeybees artificially infected with Escherichia coli exhibited increased DWV titers and developed deformed wings [67]. However, honeybees mono-inoculated with S. alvi or G. apicola demonstrated enhanced bacterial clearance from haemolymph following E. coli injection and exhibited higher levels of AMPs compared with microbiome-free bees, suggesting immune priming by these core gut community members [68]. Similarly, F. perrara induced immune priming by enhancing the production of the specific antimicrobial peptide apidaecin [16].
Feeding wounds on honeybee’s body caused by Varroa mites feeding often facilitate bacterial infiltration [69], potentially exacerbating viral infections. This can lead to dysbiosis, or a microbial imbalance, further increasing pathogen susceptibility. Tetracycline-induced dysbiosis in honeybees has been shown to promote infiltration by opportunistic bacteria and reduce lifespan following infection with Serratia marcescens pathogen [70]. Similarly, stress-induced microbiota alternations in honeybees have been linked with heightened susceptibility to Lotmaria passim [71] and worsened outcomes of DWV infections [72].
Understanding the intricate interactions within the honeybee holobiont, including Varroa mites, associated viruses, and other microbial communities, is essential for developing effective strategies to combat honeybee colony losses and ensure sustainable pollination services.
Tick–host system
Ticks are obligate hematophagous ectoparasites of terrestrial vertebrates and serve as efficient vectors of a wide range of pathogens. The colonisation, establishment and subsequent transmission of pathogens by ticks are influenced by the behaviour and composition of their vector-associated microbial communities. These cooperative or competitive interactions can directly modulate pathogen dynamics within the vector. Processes such as the production of antimicrobial compounds, signaling mechanisms or the synthesis of secondary metabolites that serve as precursors to essential metabolic pathways can facilitate pathogen development or favour the establishment of other microorganisms. Together, these microbial interactions create a permissive environment that promotes pathogen persistence and enhances transmission efficiency [73, 74].
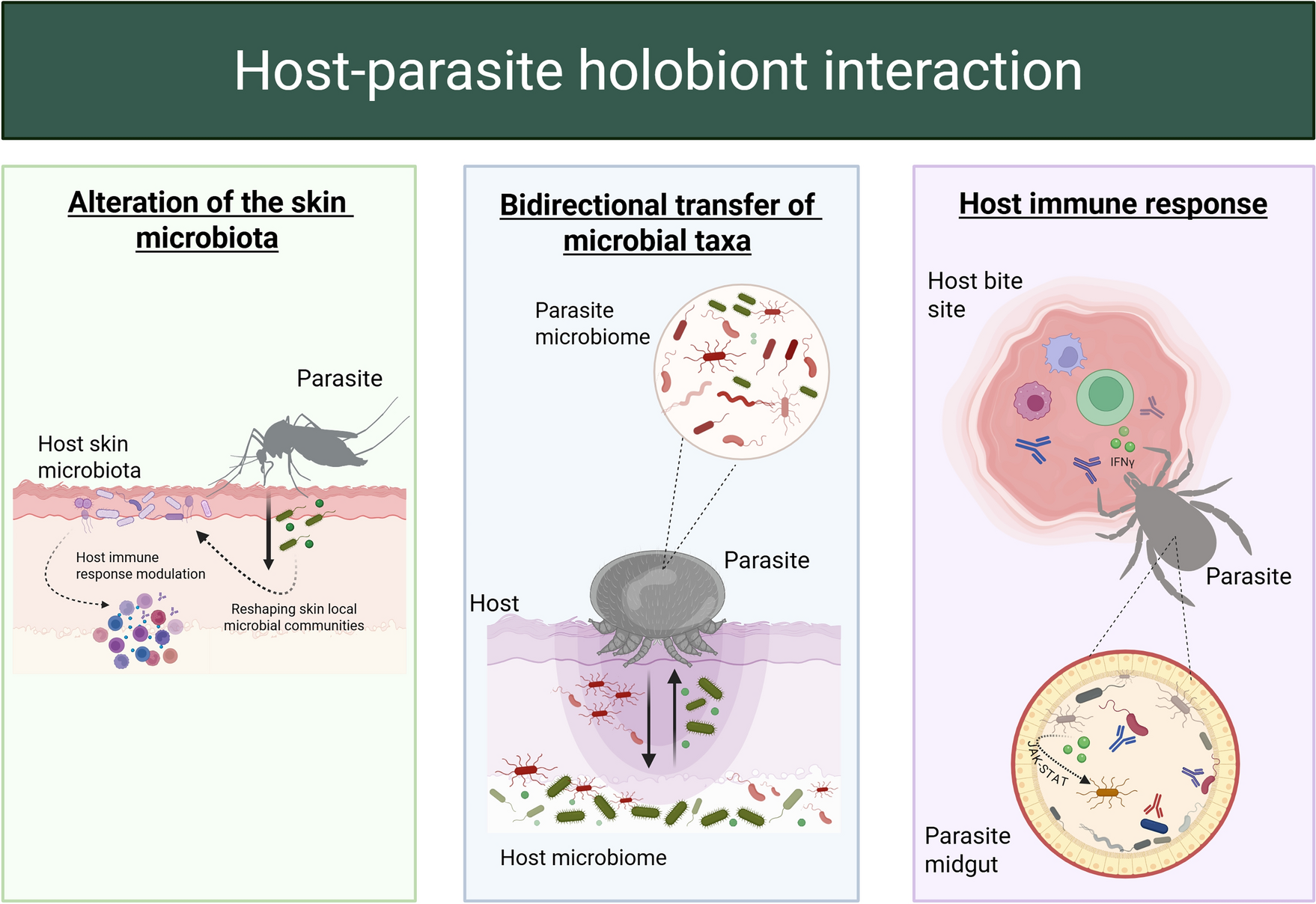
A bidirectional crosstalk is established between the tick microbiota and that of its vertebrate host, both considered holobionts (Table 1). During hematophagous feeding, the tick can acquire microorganisms from the host’s skin, metabolites, cytokines, extracellular vesicles and other immunological factors that can reconfigure its own microbiome in terms of composition, diversity and assembly. This dynamic exchange at the holobiont level suggests that the host microbiota could play an active role in pathogen colonisation and subsequent transmission, and thus in shaping vector competence, adding a new layer of complexity to the ecology of tick-borne diseases [47, 73].
The tick functions as a compartmentalised ecosystem where tissues such as the midgut, salivary glands and ovaries constitute micro-environments with specific microbial compositions and community assembly. This compartmentalisation implies that the host influence is differential in each niche, depending on acquired components such as the cutaneous microbiota, metabolites, extracellular vesicles and cytokines. The midgut, as the primary interface for host-derived components, can accentuate phenomena such as resistance to pathogen colonisation. In this context, the resident microbiota prevents the establishment of exogenous microorganisms or the overgrowth of native taxa through metabolic competition, the production of antimicrobial compounds and immune activation [73]. Consequently, the tick will function as an integrated and dynamic ecological unit where interactions between the vector, its microbiota and external stimuli (such as those derived from the host) shape its functionality and vector competence.
The conceptualisation of the holobiont framework, which integrates the host and its microbiome as a cohesive biological entity [75], has further evolved into the pathobiome paradigm, emphasising the role of microbial consortia in disease ecology, including tick-borne infections [76, 77]. Therefore, elucidating these microbe–microbe interactions is crucial for understanding their broader implications on tick physiology and vectorial capacity [45, 78, 79].
Within this framework, the host must be considered a biologically complex holobiont with considerable microbial dynamics. Before any interaction with a tick, the host holobiont is influenced by diverse and successive microbial exposures from interactions with the environment, co-infestations with other ectoparasites, the resident microbiota and contact with other hosts. This complex microbial landscape not only modulates the host’s immune and metabolic systems but also influences the response of its microbiota to vector-mediated perturbations during cross-talk. In this context, the host’s contribution to cross-talk goes far beyond passive reception; it actively conditions the nature of microbial exchange and pathogen dynamics at the tick-host interface. For instance, Boulanger et al. observed cross-talk between the Ixodes ricinus tick microbiome and mouse skin microbiome, while at the site of the tick bite, the skin microbiome was entirely altered [80].
The skin is the first physical and immunological barrier against antigens, including ectoparasites such as ticks. The host skin microbiota plays a critical role in shaping host–tick–pathogen interactions and providing surface for dynamic microbial interference, where the tick may acquire microorganisms as it feeds. This interface can be considered a starting point for understanding holobiont–holobiont interaction chains between host, tick, and pathogen [81, 82]. Notably, recent research by Boulanger et al. demonstrated that tick blood-feeding induces a profound alterations of the host skin microbiome [80]. Several bacterial families, such as Lachnospiraceae and Muribaculaceae, which are typically associated with beneficial functions in the skin and gut of mice and humans [83,84,85], were significantly depleted following tick attachment (Table 1). Several studies have explored the relationship between Ixodes spp. and their hosts, particularly focusing on the host’s influence over tick microbiomes. While findings suggest that the tick species itself is the primary determinant of microbiome composition, with minimal correlation to host microbiota, some nuances exist [86,87,88]. For example, I. scapularis and Dermacentor variabilis microbiomes remained distinct regardless of feeding on prairie voles or white-footed mice [86]. Nonetheless, other research indicates that host species can still modulate the overall microbiome composition in ticks, influencing the relative abundance of specific bacteria such as R. buchneri [89].
To further elaborate this “continuum”, during blood feeding, hematophagous vectors such as mosquitoes and ticks ingest host-derived molecules, including antibodies [46, 48, 90] and cytokines [91,92,93]. These host antibodies can interact directly with the vector gut microbiota, while cytokines influence vector immune responses. This concept is supported by experimental evidence from microbiota-targeting vaccines, which demonstrate that host antibodies can alter vector microbiome composition [46], impair vector fitness [45, 94] and reduce vector competence [48, 90]. Indeed, host immunisation with keystone taxa from the tick microbiota (highly connected microbes that shape community structure and function) induces bacterial-specific antibodies that significantly increase tick mortality [45]. These keystone taxa, widely present in the tick microbiota, likely play essential roles in microbial network stability and tick physiology [74, 78, 95]. Their targeting by host immune responses suggests a direct link between vertebrate immunity and microbiota dynamics in the tick holobiont.
A key example is the antibody response against galactose-α−1,3-galactose (α-Gal), a carbohydrate synthesised by microbial galactosyltransferases within the host and tick microbiotas. Host antibodies against α-Gal contribute to tick mortality upon blood feeding, demonstrating how vertebrate immune factors disrupt microbial-tick associations (Table 1) [45]. Beyond direct microbial targeting, host-derived antibodies also interact with tick tissues and intracellular proteins once ingested. For instance, antibodies against the protective tick antigen Bm86 – a glycoprotein located in the gut epithelial cells [96] – bind to intestinal cell surfaces, causing cell lysis and reducing female reproductive success [97].
Recent advances in anti-microbiota vaccines have demonstrated their ability to selectively target key bacterial taxa within the tick microbiota, altering microbial composition and affecting tick fitness [45, 46]. Whereas Escherichia–Shigella was identified as a central component of tick microbial communities and Enterobacteriaceae family was found to be a keystone taxon, the Escherichia coli bacterium, fulfilling both roles, was targeted through host immunisation [45]. Mice immunised with live E. coli developed high titers of E. coli-specific immunoglobulin (Ig) M and IgG, which negatively correlated with Escherichia–Shigella abundance in ticks [46]. Ticks feeding on E. coli-immunised mice exhibited significantly increased engorgement weight compared with those feeding on mock-immunised controls, highlighting immunisation-driven microbiota modulation [45, 46]. Moreover, high tick mortality was observed when feeding on hosts with elevated IgM and IgG levels against α-Gal [45].
Ticks I. scapularis, feeding on mammalian hosts infected with the Lyme disease-causing bacteria B. burgdorferi, responded to the interferon γ (IFN-γ) cytokine derived from the hosts. The ticks ingesting IFN-γ during feeding showed enhanced expression of the signaling transducer activator of transcription (STAT) factor, which is necessary for activating inducible guanosine triphosphatase (IGTPase). This activation induced the production of antimicrobial peptide Dae2, which helped to kill the Borrelia pathogen, resulting in a significant reduction in the B. burgdorferi survival in the ticks [73, 91]. Similarly, in another experiment, the ingestion of natural antibodies reduced the spirochete B. burgdorferi burden within feeding I. scapularis nymphs [98].
This immunological-microbial interplay extends to pathogen control (Table 1). Host antibodies can persist in the tick midgut for varying durations, influencing both microbiota composition and pathogen transmission [99]. Empirical evidence shows that vertebrate antibodies can target vector-borne pathogens within ticks [100]. A notable example is B. burgdorferi, where anti-BBA52 antibodies, directed against an outer membrane protein preferentially expressed in feeding ticks, block spirochete transmission to murine hosts without reducing bacterial loads in the tick gut [100].
These intricate holobiont–holobiont interactions between the host and tick microbiotas are pivotal in shaping vector competence, pathogen transmission and overall tick fitness. The reciprocal influence of host immune responses on tick microbial communities, combined with the impact of tick microbiota on host–pathogen dynamics, emphasises the interconnectedness of both holobionts. Understanding these bidirectional interactions opens avenues for targeted interventions, such as anti-microbiota vaccines, that could disrupt this loop, potentially reducing tick-borne pathogen transmission.
Bat fly–bat system
Bat species are important reservoirs for a wide range of pathogens and are reported to harbour a greater diversity of viruses per species than rodents [101, 102]. Among many ectoparasites that infest bats are ticks (Acari: Argasidae and Ixodidae), mites (Mesostigmata: Spinturnicidae and Macronyssidae), fleas (Siphonaptera: Ischnopsyllidae), bugs (Hemiptera: Cimicidae and Polyctenidae), earwigs (Dermaptera: Arixeniidae) and, most notably, bat flies (Diptera: Nycteribiidae and Streblidae), which represent the most prevalent and specialised group of bat ectoparasites [103, 104].
Bat flies exhibit a blood-feeding dietary strategy, remaining on their bat hosts throughout their entire lives [105]. They exhibit remarkable morphological and behavioural adaptations well-suited to their parasitic lifestyle. Bat flies frequently experience co-infestation, where multiple ectoparasite species inhabit the same host simultaneously, facilitating the exchange of microorganisms within diverse microbiomes. These coexistences generate a dynamic microbial interface between holobionts, enabling interspecific microbial exchange and modulating immunological and metabolic responses in both the vector and the host. These processes exemplify a broader holobiont–holobiont dynamic, similar to that previously described in the tick–host system, where successive microbial exposures from environmental sources or other ectoparasites shape the host’s immune and metabolic landscape prior to any interaction with a specific vector’s microbiota (Table 1).
Additionally, bat hosts living in colonies further promote microbial transmission through social behaviours such as grooming, which influences the spatial distribution and ecological niche specialisation of bat flies (Fig. 3) [106]. These collective behaviours significantly increase pre-exposure to diverse microbial consortia, both in composition and assembly, which reshapes the bat microbiome before any direct interaction with the vector. A directed crosstalk arises between host and ectoparasite, where bat-derived microbial and immunological signals can actively modulate the structure and function of the microbial community within the bat fly holobiont.
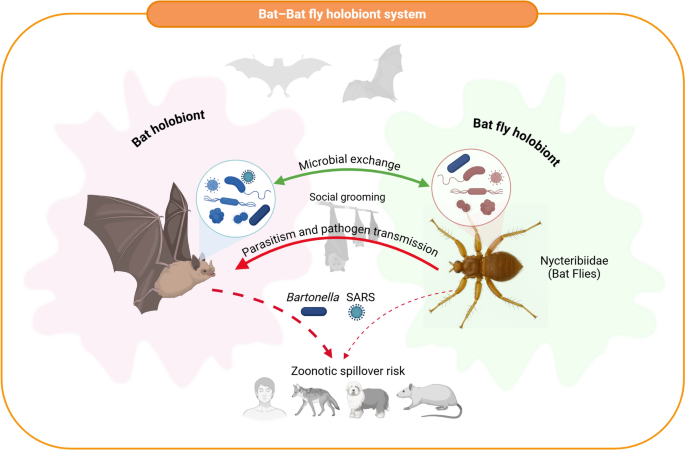
Schematic illustrating the interactions between a bat holobiont and a Nycteribiidae bat fly holobiont. Each holobiont consists of its respective host or ectoparasite along with associated microbial communities. Microbial exchange occurs between bats and their bat flies, facilitated by close physical contact and social grooming behaviours within bat colonies (silhouetted above). Parasitism and pathogen transmission pathways are illustrated by red arrows, highlighting the movement of key zoonotic agents, such as Bartonella (bacterium) and severe acute respiratory syndrome (SARS)-like coronaviruses (virus), between bat flies, bats and potential secondary mammalian hosts. The diagram highlights zoonotic spillover risk to humans, domestic dogs and rodents, representing primary exposure routes for these pathogens. This conceptual model underscores the ecological complexity of bat–bat fly holobiont systems and their importance for One Health frameworks and emerging infectious disease surveillance
Owing to their diverse feeding guilds (insectivores, frugivorous, nectarivores, carnivores and sanguivorous diets) [107, 108], bats can act as vectors for pathogen transmission to other organisms, including humans, creating complex quadripartite interactions. In Nigeria, for example, people enter caves to capture fruit bats (Rousettus aegyptiacus) infested by bat-associated nycteribiid flies (Eucampsipoda africana); the bats are then prepared and consumed during a traditional festival [109]. Additionally, bat faeces are frequently collected and used as fertiliser for vegetable farms [110]. Notably, individuals engaged in these activities have been found to be seropositive for Candidatus Bartonella rousetti, suggesting potential zoonotic transmission route via bat flies [109]. Moreover, another study identified bat flies as potential vector of Bartonella spp., capable of transmitting the bacteria to new host organisms [111], such as rodents, carnivores and herbivores, in which Bartonella had already been detected [112].
Overall, these findings suggest that bats, ectoparasites and other hosts form a dynamic network of microbial exchange, where each organism functions as an integrated unit with its microbiota. The potential transfer of microbial taxa between bat flies and vertebrate hosts exemplifies a holobiont interaction, in which microbial communities are not only transmitted but can also adapt and reconfigure in new ecological niches, influencing the physiology, immunity and pathogen susceptibility of the new host.
Some Bartonella species are known to cause human diseases, including trench fever (Bartonella quintana), cat scratch disease (Bartonella henselae), Carrion’s disease (Bartonella baciliformis) and various forms of bacteraemia or chronic infections (Bartonella elizabethae) [113]. Interestingly, similar Bartonella genotypes have been identified in the bats from Georgia, sera samples from the forest workers in Poland, and dogs in Thailand [114]. Likewise, Candidatus Bartonella mayotimonensis was detected in the aortic valve of a patient with endocarditis in the USA as well as in bats of Myotis lucifugus and Myotis grisescens [111] (Table 1).
To date, research on the bat fly microbiome remains limited, with most studies focusing on two bacterial groups: Bartonella and Arsenophonus-like organisms (ALOs), which include species such as Arsenophonus, Aschnera and Riesia. Bartonella, an intracellular parasitic bacterium linked to zoonotic diseases [28] has also been detected in ticks (Argasidae: Ornithodoros spp., Carios spp.) [115, 116], suggesting the potential for co-infection or interactions between bacterial species. In contrast, ALOs are primary endosymbiotic microorganisms that provide essential nutrients, such as vitamin B, to their bat-fly hosts, as the vertebrate blood they feed on is deficient in this nutrient. While Bartonella is thought to have co-evolved with bats and bat flies [118], interactions between ALOs and their hosts – bat flies – remain undetected (Table 1) [119].
Bats are often linked to the transmission of various zoonotic diseases to humans, including rabies [120], Ebola haemorrhagic fever [121], Nipah viral encephalitis [122] and numerous species of coronaviruses [140], such as severe acute respiratory syndrome (SARS) [124] and, most recently, the coronavirus disease 2019 (COVID-19) virus [125]. Even ectoparasites, such as bat flies, can harbour various virus species, with the most dominant being narmaviruses, reoviruses and sebemo-like viruses [123].
Analysing vector–host holobionts through the perspective of both global interaction and compartmentalisation allows for a broader interpretation of microbial interactions among microorganisms of different aetiologies and pathogenesis mechanisms. In this context, considering microbial tropism, the dynamic interaction between microorganisms such as viruses and bacteria can be understood through their coordinated influence on colonisation, persistence and transmission. These microbial agents can form complex virus–bacteria associations, where they interact in ways that can influence their survival, replication and transmission. For example, certain bacteria may enhance viral stability or facilitate infection by weakening the host’s immune response. Conversely, some viruses can alter bacterial behaviour, increasing their pathogenicity or enabling co-infections. Such interactions can occur within a shared host, such as bats or their ectoparasites, potentially amplifying the risk of cross-species transmission to humans. Understanding these complex relationships is crucial for evaluating the dynamics of zoonotic disease emergence and developing effective control strategies.
From a holobiont–holobiont perspective, the bat–bat fly system exemplifies a biologically integrated network of microbial community exchange, reconfiguration and co-evolution. Microbiome-level interactions between bats and their ectoparasites are characterised as bidirectional, mediated by shared environments, feeding behaviours and social dynamics. This framework highlights the importance of considering zoonotic emergence not only from the perspective of pathogen spillover but also as a product of microbial co-evolution and ecological connectivity at the holobiont level.
Mosquito–host system
Mosquitoes are primary vectors of some of the world’s deadliest diseases, including malaria, dengue and lymphatic filariasis [124, 125]. According to the World Health Organization (WHO) [126], malaria alone causes over 650,000 deaths annually, primarily in tropical and subtropical regions. While traditional research has focused on direct pathogen–vector interactions, particularly mosquito physiology, immunity and the parasite–host relationship, recent evidence suggests that these interactions occur within a broader ecological and microbial framework.
Both mosquitoes and their vertebrate hosts function as interconnected holobionts, where the microbiota plays a fundamental role in immune modulation, pathogen transmission and vector competence [127]. Guéran et al. emphasised that an arthropod vector should no longer be viewed as an isolated organism but rather as an interactive system (vector holobiont) in which the vector and its microbiota operate as an integrated unit [128]. While this perspective has shifted research focus towards understanding the influence of mosquito microbiome on pathogen transmission, we propose that the holobiont model must be further expanded – to include the impact of vertebrate host microbiota and immune factors on mosquitoes.
The mosquito microbiota, residing in the gut, salivary glands and reproductive organs, plays a crucial role in shaping vector fitness, immune responses and pathogen susceptibility (Table 1) [129]. Several studies have identified core bacterial taxa shared across mosquito species, while others exhibit species- and organ-specific microbial compositions. Mancini et al. analysed nine mosquito species from the genera Anopheles, Aedes and Culex and found that Serratia was present in the salivary glands of all species [130]. Other shared bacterial taxa included Escherichia–Shigella, Pantoea, Acetobacter, Sphingomonas, Burkholderia and Cupriavidus. Accoti et al. identified Proteobacteria and Bacteroidetes as dominant phyla in Anopheles gambiae and Anopheles stephensi microbiota, with Serratia, Elizabethkingia, Acinetobacter and Comamonas as the most abundant midgut genera [131]. Onyango et al. reported that Aedes albopictus midgut and saliva contain Bacteroidetes and Proteobacteria as core phyla, along with Elizabethkingia, Pseudomonas, Sphingomonas and Wolbachia [132].
When mosquitoes take a blood meal, they ingest not only pathogens but also vertebrate immune molecules and microbiota, which can alter their gut microbial community and immune responses. Hematophagous insects, including Anopheles mosquitoes, have evolved mechanisms to regulate interactions between ingested blood, gut microbiota and immune responses. One such mechanism is the formation of the peritrophic matrix, a chitinous, semi-permeable barrier that encapsulates the blood meal, preventing direct contact between blood components and the gut epithelium (Table 1) [133]. In addition, the immunomodulatory peroxidase (IMPer), secreted by midgut epithelial cells, cross-links mucin proteins to further limit permeability to immune elicitors and protect resident microbiota from immune attack [134]. Despite these protective barriers, host-derived immune molecules, including cytokines and antibodies, can persist in mosquitoes post feeding. Studies have detected vertebrate host antibodies adhering to the midgut epithelium and even present in the haemocoel of Anopheles and Culex mosquitoes [135, 136]. Interestingly, Meyers et al. demonstrated that IgG translocation varies across mosquito species, with anti-glutamate-gated chloride channel (anti-GluCl) IgG detected in An. gambiae but not in Ae. aegypti or Culex tarsalis, suggesting species-specific differences in midgut permeability to host immune factors [137].
The peritrophic matrix is suggested to function not only as a barrier for immune elicitors but also contributes to the selective retention of symbiotic and commensal bacteria, allowing certain bacterial species to persist within the midgut while excluding others [138]. This selective filtering function may influence how host-derived microbiota interacts with resident mosquito microbiota, potentially reshaping microbial composition after each blood meal. The presence of host-derived immunoglobulins raises intriguing questions regarding their impact on mosquito physiology, microbiota composition and vector competence (Table 1). Recent findings by Aželytė et al. indicate that natural antibodies, such as anti-α-Gal IgY, can shape mosquito gut microbiota. Their study showed that Culex pipiens mosquitoes feeding on birds immunised against E. coli O86:B7 (which expresses high levels of α-Gal) exhibited altered microbiota composition. This shift in microbial diversity was associated with reduced Plasmodium development, suggesting that host-derived antibodies can influence mosquito microbiota and indirectly modulate vector competence [90].
Similarly, transforming growth factor-β1 (TGF-β1), a cytokine present in mammalian blood, remains active post ingestion and triggers MEK-ERK signaling in mosquito cells (Table 1) [139]. Orthologous TGF-β receptors and Smad signaling proteins have been identified in Anopheles mosquitoes, indicating that vertebrate immune cytokines may actively regulate mosquito immune responses [93, 140]. This highlights the concept that mosquito immunity is not only shaped by its microbiome but also by vertebrate immune factors [73], reinforcing the cross-talk holobiont concept.
The potential impact of these immune molecules on mosquito microbiota remains an emerging area of research. Cytokines, such as TGF-β1, may influence the balance of gut microbial species by modifying the physiological conditions of the midgut, such as pH levels, antimicrobial peptide production or epithelial integrity [138]. These indirect effects could promote the growth of specific bacterial taxa while suppressing others, thereby altering microbial interactions that influence pathogen survival within the mosquito [141]. Future studies should investigate whether host immune molecules can persist within mosquito tissues long enough to have lasting effects on microbial homeostasis and vector competence.
Vertebrate host-associated microbiota plays a crucial role in mosquito attraction by releasing volatile organic compounds [138]. Takken and Verhulst emphasise that mosquitoes rely on host skin microbiota-derived volatiles, with bacteria such as Bacillus, Brevibacterium, Corynebacterium and Staphylococcus producing attractant compounds, while Pseudomonas suppresses them [142]. This dynamic interaction suggests a co-evolutionary relationship, where mosquito host-seeking behaviour has been shaped not only by vertebrate hosts but also by their associated microbiomes, reinforcing the role of microbial-mediated chemical communication in mosquito–host interactions. Additionally, Byrd et al. highlight the crosstalk between the immune system and the skin microbiota, demonstrating how resident microorganisms can influence immune responses, promoting either immune tolerance or inflammation [143]. Specific microbial species can strengthen immune homeostasis, while others activate defensive pathways, impacting skin barrier integrity and systemic immunity.
Blood-feeding mosquitoes repeatedly interact with the skin, inoculating saliva during feeding and serving as persistent immune triggers, potentially reshaping local microbial communities and immune responses with each feeding event. Accoti et al. demonstrated that mosquitoes can transmit bacteria present in their saliva into vertebrate hosts during blood feeding [131]. Using fluorescently labelled S. marcescens, authors showed that this bacterium was introduced into mice through mosquito bites and later detected in multiple organs, including the liver, lungs, kidneys, spleen, brain and heart. These findings suggest that mosquitoes act as vectors not only for parasites and viruses but also for bacteria, which can be found as part of their microbiota, suggesting a deeper co-evolutionary relationship between mosquitoes, vertebrate hosts and their microbiota. The persistent exposure to mosquito-inoculated microbes may further shape vertebrate immune responses, potentially influencing host susceptibility to infections and immune homeostasis.
The microbiota of mosquitoes and vertebrate hosts acts as a selective force that influences pathogen transmission. Over evolutionary time, selection pressures may have favoured mosquito-associated microbial communities to either facilitate or inhibit pathogen development [144]. Meanwhile, pathogen-induced microbiota shifts of vertebrate hosts could present an adaptive strategy that enhances mosquito attraction. Research has shown that mosquitoes are more attracted to, and more likely to bite, infected hosts rather than uninfected ones. Female mosquitoes can detect the volatile organic compounds (VOCs) produced by host microbiota present in the skin [145].
Environmental factors, such as climate, breeding sites and host diversity, further shape these interactions. Mosquito microbiota is acquired from larval habitats, meaning that ecological disturbances – such as habitat destruction, pollution or climate change – may influence the microbial diversity of mosquito populations. Temporal environmental changes led to variations in the bacterial community structure of mosquitoes, potentially contributing to their physiological changes [146]. Similarly, shifts in vertebrate microbiota may appear owing to dietary changes or environmental stressors, which could further alter mosquito feeding preferences and even reinforce the transmission efficiency of parasites [147, 148]. Understanding how mosquitoes acquire their microbiota and how environmental pressures shape microbial communities is critical for predicting future changes in vector competence and disease dynamics.
The co-evolution of mosquito and vertebrate microbiomes represents a paradigm shift in vector biology. Future research should aim to decipher the long-term evolutionary consequences of microbial exchange, assessing the stability of mosquito microbiota across multiple feeding cycles, and exploring how microbiome-targeted strategies can be effectively integrated into vector control strategies. Innovative approaches that combine genetic manipulation, microbiome engineering, and ecological interventions may provide the next generation of sustainable malaria and vector-borne disease control strategies. The growing recognition of mosquito microbiota as a key determinant of pathogen transmission suggests that holobiont-based strategies could complement existing methods such as insecticide use, genetic modifications and habitat management, offering a more holistic, durable approach to vector control.
In summary, the data in Table 1 emphasise that microbial interactions between holobionts encompass not only direct transfer events but also integrated modulatory processes (environmental, sequential or niche-mediated) that actively contribute to remodelling symbiotic assemblages in host-ectoparasite systems.
Challenges and future directions
Over the past decade, high-throughput sequencing and advanced bioinformatics tools have revolutionised our understanding of microbial communities, revealing insights into their diversity, ecology, evolution and dynamics [7]. Despite these advancements, significant knowledge gaps remain, as researchers encounter unresolved questions across various studies [10]. For example, what are the functions of key microbial taxa and how do they affect pathogen biology and physiology? How do microbial communities interact with each other and with their host environments? What are the mechanisms by which beneficial microbes suppress or promote pathogen virulence?
While many studies have advanced our understanding of holobiont systems, there is a pressing need for modern computational methods, statistical analysis and advanced bioinformatics to be integrated into future research. In particular, investigating holobiont–holobiont interactions could greatly enhance our understanding of host health, ecological stability and evolutionary dynamics [149].
In both human and veterinary medicine, these innovative approaches have the potential to revolutionise disease prevention, control and diagnostics. Identifying key microbial taxa could lead to development of microbiota-based vaccines, precise microbiome manipulations and targeted interventions to disrupt pathogen transmission [150]. For instance, Mateos-Hernández et al. [45, 46] and Wu-Chuang et al. [47, 48] introduced anti-tick microbiota vaccines by targeting keystone taxa within tick microbial assemblies, thereby reducing pathogen loads transmitted by these blood-sucking ectoparasites. Similarly, Aželytė et al. conducted an initial study of anti-microbiota mosquito vaccine, which aimed to reduce avian malaria infection [90]. Beyond the advantages of anti-microbiota vaccines, microbiome manipulation strategies have the potential to address the growing problem of antibiotic resistance. Microbiome-based therapies could help assess disease susceptibility, manage antibiotic resistance and design treatments that either enhance beneficial microbes or suppress harmful pathogens [150]. One promising para-transgenic strategy is the release of Wolbachia-infected Ae. aegypti, pioneered by the World Mosquito Program, which demonstrably lowers transmission of DENV, ZIKV, yellow fever and CHIKV; similar symbiont-based modifications of other ectoparasites could open novel avenues for disease control [151].
A promising strategy for controlling vector-borne diseases is the development of immunobiotics – specific bacterial strains that stimulate the immune systems of both hosts and vectors [73]. The goal is to enhance host defences while indirectly influencing vector competence by transferring immune molecules (e.g. cytokines or natural antibodies) during blood feeding of the vector. This cross-species immune activation occurs when host-derived immune molecules interact with the vector’s immune system by binding to receptors, modulating signaling pathways and ultimately affecting pathogen transmission. Rather than providing direct protection to the host, these immune molecules enter the vector and impact the pathogen within its body [73].
Although manipulating the microbiome could alter the holobiont’s immune system, our goal is not to destabilise it. Instead, we seek to modulate microbial interactions to promote pathogen neutralisation, reduce virulence and pathogenicity and influence their ecological translocation into target compartments or niches that favour tropism or facilitate transmission. To this end, manipulation strategies could focus on functional sub-communities within specific ecological niches, allowing for localised, non-disruptive interventions. Microbial taxa that exert neither direct nor indirect influence on pathogen dynamics can thus remain unaltered, preserving the integrity of broader microbial networks and the overall architecture of the holobiont.
Importantly, targeted interventions allow for the preservation of stable or neutral microbial components, ensuring that the integrity of macro-ecological structures remains intact. As holobionts and their associated microbiota evolve (through taxonomic shifts, functional reconfiguration and changing interactions with each other), strategies must be continually updated to maintain their adaptability and ecological viability.