From the kitchen to the brain, a new review reveals how everyday meals shape AGE–RAGE pathways that fuel inflammation and neurodegeneration, and why changing diet could slow the damage.
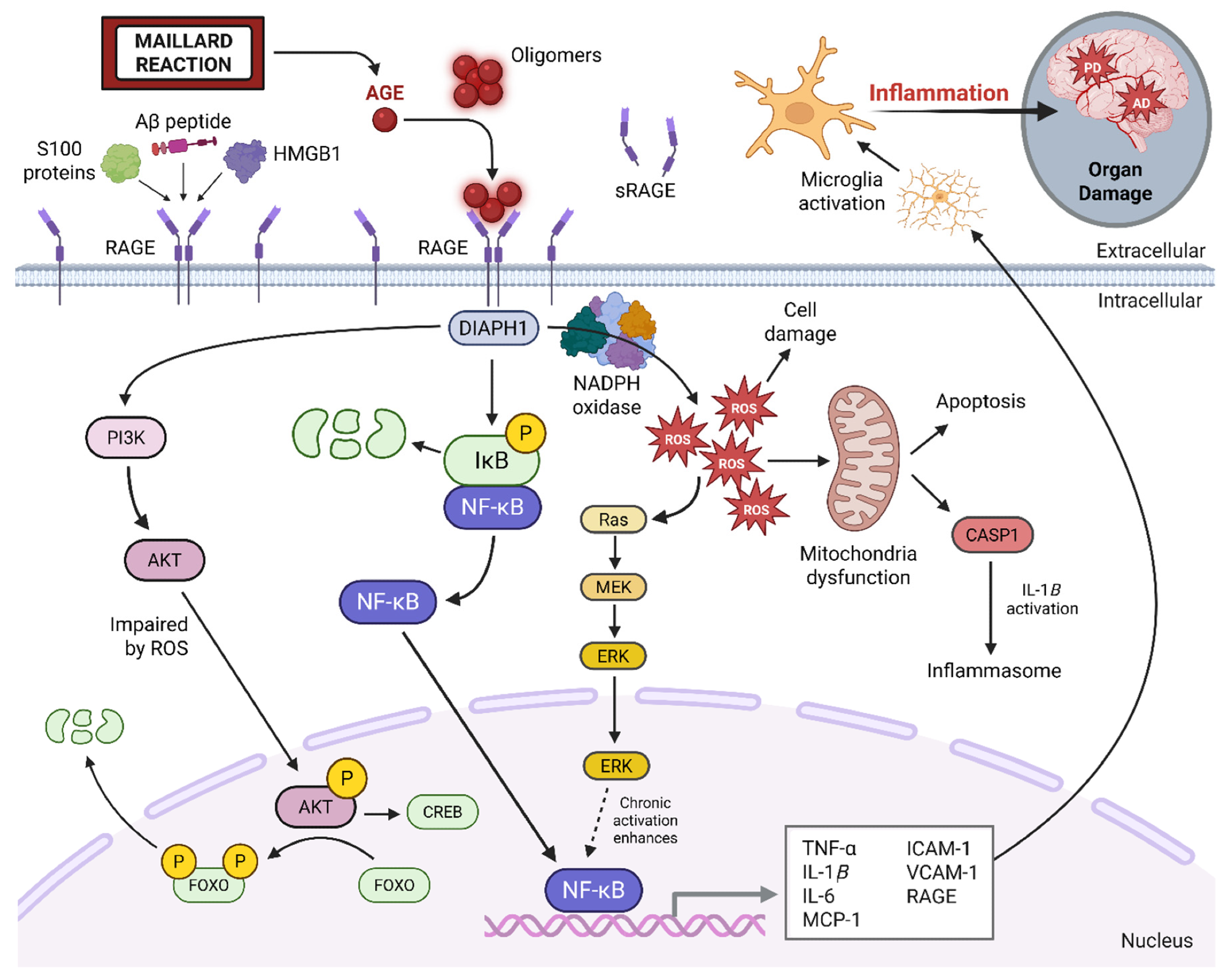
AGE–RAGE signaling axis. The AGE–RAGE signaling pathway is triggered when advanced glycation end products (AGEs) and other ligands (e.g., Aβ, S100, HMGB1) bind to the RAGE receptor. This interaction activates intracellular signaling through DIAPH1, leading to NF-κB activation and increased expression of pro-inflammatory genes. The pathway also increases reactive oxygen species (ROS) production, causing mitochondrial dysfunction and promoting further AGE formation. This creates a damaging feedback loop that impairs cell function and promotes chronic inflammation. Soluble RAGE (sRAGE) serves as a natural decoy, reducing pathway activation. Image was created using BioRender.
In a recent NeuroSci review, researchers examined how dietary sources and food preparation methods contribute to signaling pathways associated with neurodegenerative illness. They found that these pathways play a central role in driving neurodegeneration and chronic inflammation, highlighting dietary AGE restriction as a therapeutic or preventive strategy for neuroinflammatory and neurodegenerative disorders.
AGE, RAGE Interactions and Neurodegeneration
Neurodegenerative diseases (NDDs) affect over 9 million Americans and neurological disorders affect about 15% of the global population. Myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS), which shares features with post-coronavirus disease 2019 (COVID-19) syndrome, or long COVID, is one such condition. Both are characterized by systemic dysfunction, where neuroinflammation, elevated reactive oxygen species (ROS), and prolonged microglial activation are central to the pathology.
Recent research highlights advanced glycation end-products (AGEs) and their receptor (RAGE) as key contributors to these processes. When AGEs bind to RAGE, they activate signaling cascades that increase ROS, impair mitochondrial function, and stimulate NF-κB, a transcription factor driving pro-inflammatory gene expression. This feedback loop sustains oxidative stress and chronic inflammation, both of which are hallmarks of aging and progressive neurodegeneration.
These mechanisms link AGE–RAGE signaling to conditions ranging from Alzheimer’s disease to ME/CFS and long COVID.
Sources and Absorption of AGEs
AGEs form through the Maillard reaction, in which sugars react with amino acids, particularly lysine and arginine, under heat. While responsible for the browning of grilled or roasted foods, this process produces irreversible compounds that can damage proteins, lipids, and nucleic acids.
Within the body, AGEs form slowly under normal conditions and are typically cleared by proteasomes, lysosomes, and the kidneys. However, clearance efficiency declines with age, diabetes, and chronic disease, allowing AGEs to accumulate.
Endogenously, AGE production increases under hyperglycemia, oxidative stress, and lipid peroxidation, all of which are common in Western dietary patterns.
Exogenous AGEs are abundant in meats, cheeses, and processed foods, particularly those prepared using dry, high-heat cooking methods. Moist-heat cooking, like boiling or steaming, produces fewer AGEs. Although only 10–30% of dietary AGEs are absorbed, they still significantly elevate circulating levels, depositing in organs such as the liver, kidneys, and brain.
Diet, Environment, and AGE Accumulation
The Western diet, characterized by its high consumption of processed meats, refined sugars, and saturated fats, promotes AGE formation. Refined carbohydrates and fructose amplify post-meal glucose spikes, accelerating glycation.
Fructose is more reactive than glucose and accelerates protein crosslinking, oxidative stress, and inflammation. Saturated fats add to the problem by impairing mitochondrial function and weakening antioxidant defenses.
Mediterranean and plant-based diets, emphasizing whole foods and low-heat cooking, reduce AGE intake and provide antioxidants that neutralize intermediates and inhibit glycation.
Beyond diet, environmental contaminants such as cadmium, mercury, pesticides, and food additives may also contribute to AGE production and RAGE activation; however, the review notes that direct mechanistic evidence linking many additives to AGE–RAGE activation is limited and warrants further study.
Heavy metals impair detoxification pathways, disrupt mitochondria, and reduce kidney clearance of AGEs, compounding systemic accumulation. Synthetic dyes, preservatives, and emulsifiers in ultra-processed foods can heighten oxidative stress, weaken gut barrier function, and trigger immune responses, further fueling AGE–RAGE signaling.
AGE, RAGE Signaling and Neuroinflammation
AGEs exert much of their damage through binding to RAGE, which is widely expressed in vascular, neural, and immune tissues. This interaction activates NF-κB, increases ROS production, and compromises mitochondrial function, creating a cycle of inflammation and oxidative stress.
In the brain, RAGE signaling contributes to microglial overactivation, amyloid plaque buildup, and neuronal injury, linking diabetes and Alzheimer’s disease.
Post-viral syndromes such as ME/CFS and long COVID are discussed as conditions in which AGE–RAGE signaling may play a contributory role, based on emerging mechanistic evidence, including RAGE upregulation and convergence on NF-κB pathways.
Viral infections upregulate RAGE expression, intensifying inflammatory cascades. In COVID-19, RAGE is highly expressed in lung and vascular tissues, where it drives cytokine storms, endothelial dysfunction, and mitochondrial stress.
Persistent viral fragments, combined with impaired renal clearance, may prolong AGE accumulation, leading to neuroinflammation, blood–brain barrier disruption, and cognitive decline.
Strategies for Reducing AGE Burden
Pharmacological inhibitors of the AGE–RAGE pathway have shown mixed results. Agents such as azeliragon and FPS-ZM1 demonstrated neuroprotective effects in preclinical models but achieved limited success in clinical trials.
Other approaches, such as soluble RAGE (sRAGE) decoys, are still largely experimental. This underlines the importance of lifestyle-based strategies, which are both practical and effective.
Reducing AGE intake can be achieved through simple changes. Low-heat cooking methods, such as boiling or steaming, minimize AGE formation. Marinating meats in acidic solutions can also inhibit the Maillard reaction.
Herbs and spices such as cinnamon, oregano, and cloves add antioxidant protection, helping to counteract glycation. Avoiding processed foods, synthetic additives, and environmental toxins further reduces systemic burden.
Dietary interventions already show promise. AGE-restricted diets lower circulating AGE levels, are associated with improved vascular function, and may help reduce neuroinflammation, but the authors call for rigorous trials in aging and post-viral cohorts to establish causal benefits.
Anti-inflammatory patterns such as the Mediterranean or plant-based diets consistently demonstrate protective effects on metabolic and cognitive health.
Conclusions and Future Directions
AGE–RAGE signaling plays a central role in driving inflammation, oxidative stress, and mitochondrial dysfunction, contributing to conditions such as Alzheimer’s disease, ME/CFS, long COVID, and other neurodegenerative disorders.
Unlike fixed genetic or environmental risk factors, dietary AGEs are modifiable. This makes food choices and cooking methods powerful tools for prevention and intervention.
Future research should prioritize dietary intervention trials in aging and post-viral populations, assessing the impact of AGE restriction on inflammation, cognitive outcomes, and metabolic balance.
Molecular profiling, imaging, and biomarker development could further clarify individual susceptibility. Integrating AGE awareness into public health guidelines, nutritional education, and food labeling would empower individuals to make informed decisions.
Journal reference:
- From Fork to Brain: The Role of AGE,RAGE Signaling and the Western Diet in Neurodegenerative Disease. Pomroy, H.J., Mote, A., Mathew, S., Chanasseril, S., Lu, V., Cheema, A.K. NeuroSci (2025). DOI: 10.3390/neurosci6030089, https://www.mdpi.com/2673-4087/6/3/89