The use of AF therapy did not appear to significantly affect the rate of change in mPAP in the population of patients with ILD requiring lung transplantation. Additionally, AF usage was not found to augment the effects of PH therapy on mPAP change. Age was associated with a faster mPAP rate of change, and PH therapy was associated with a slower or negative mPAP rate of change.
The increasing prevalence of PH in patients with IPF as the disease progresses suggests that fibroproliferative damage of the lung parenchyma leads to vascular dysfunction that contributes to PH development [6, 8, 16, 17]. As such, we hypothesized that AF therapy would attenuate the increase in mPAP change over time by mitigating the progression of fibrosis in patients with ILD. However, our findings did not support that hypothesis. However, it is gratifying to note that the use of PH therapy did ameliorate the rise in mPAP attesting to the hemodynamic benefit of these agents in patients with ILD. Notably, the prevalence of PH was quite high (88.2%) at the time of transplant, which is indicative that the development of PH in ILD is a marker of end-stage disease.
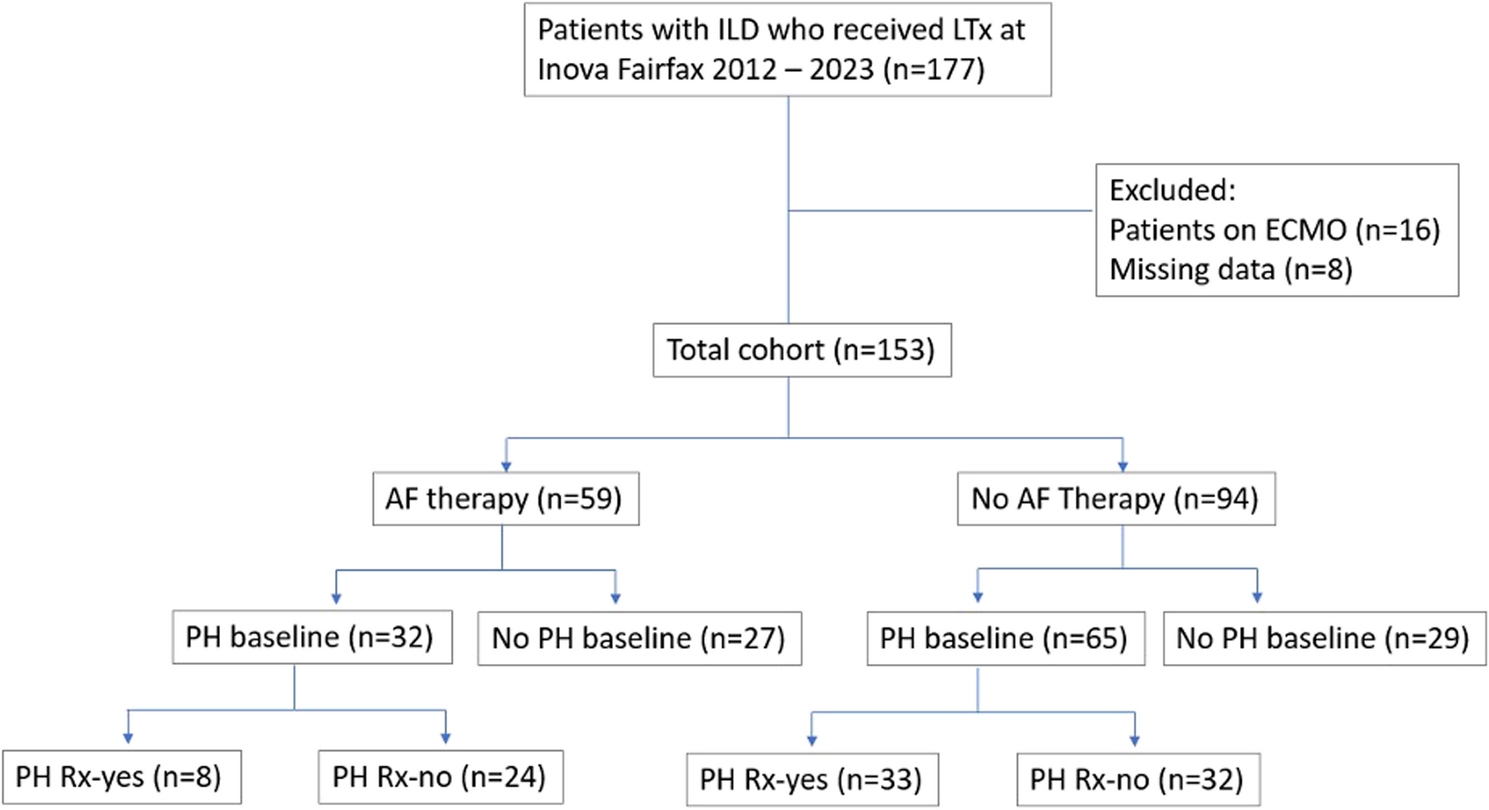
There are several limitations to this study. The power of the findings is limited by the modest patient sample size. Although we report the first RHC data performed at the time of lung transplant evaluation as “baseline”, these patients had advanced disease and likely had occult PH for a variable period of time prior to this point. The AF and no AF groups had significant differences in baseline age, gender, FVC, FEV1, 6MWD and mPAP. Due to the significant differences in the groups, it is challenging to draw meaningful conclusions from the data, as any differences in outcomes could be related to differences in the baseline parameters. Specifically, our failure to find any significant differences in the change in mPAP during the study period could be due to baseline differences between the groups, rather than the antifibrotic medication. Additionally, we were unable to control for the possibility that patients developed other reasons for a change in their PA pressures during the study period, such as thromboembolic disease or left ventricular diastolic dysfunction. However, patients were monitored very closely pretransplant for the development of any such comorbidities.
The retrospective nature of this study introduced challenges in controlling measurement conditions. The mPAP measurements were recorded under different circumstances: specifically, the mPAPbaseline was measured in the cardiac catheterization lab under local anesthesia, whereas the mPAPfollow−up was measured intraoperatively under general anesthesia. Local anesthesia administration is targeted and doesn’t involve mechanical ventilation, which helps avoid pulmonary pressure elevation. In contrast, the process of general anesthesia (including induction, maintenance, and emergence) has unpredictable effects on pulmonary hemodynamics due to direct medication effects, hypoxemia, body temperature fluctuations, cardiac preload changes, and mechanical ventilation [18]. Ultimately, it is possible that general anesthesia induction agents may have lowered the mPAP readings, resulting in an underestimation of the mPAPfollow−up values. In any event, all patients were subjected to the same circumstances and the difference in the two groups would therefore be negated by this systemic “error”. Despite this, there remains a risk of bias due to the different measurement conditions at baseline and follow up, which may limit the robustness of our conclusions.
The time between mPAP readings varied broadly between patients, necessitating the mPAP change to be reported in mmHg per month. The time between the baseline and follow-up RHC measurements is variable and a “snapshot” in time for each individual patient. Thus, the calculated rate of change might not reflect the rate of change at other points in the patients’ disease course. Unfortunately, CO and PCWP were not obtained in the operating room, thus rendering the PVR incalculable. Indeed, it is conceivable that AF therapy influenced the pulmonary vasculature that impacted the CO more than the mPAP, such that there might have been changes in the PVR that we were unable to ascertain. It is also possible that any effects on the pulmonary vasculature might take longer to emerge than this current analysis allowed. Lastly, results may be skewed by selection bias, as LTx is reserved for end-stage disease only, meaning that findings could differ for IPF or ILD patients with earlier stages of disease [6].
As PH therapy and age were found to correlate significantly with the rate of mPAP change over time, these factors likely confounded the results. Before conclusive statements are made about the therapeutic effectiveness of AF regarding PH development, randomized controlled trials with standardized intervals for mPAP readings and other outcome measurements are necessary. The cohort for analysis should include patients across different severities of disease progression and different age groups. The presence of other treatments, particularly PH therapy, must also be considered as confounding factors. As the development of PH in the context of lung fibrosis is incompletely understood, the presence of other comorbidities related to ILD may also be worth noting. For example, IPF patients have an increased prevalence of thromboembolic disease, coronary artery disease, and sleep-disordered breathing, all of which may affect underlying PH development [17]. As PH development and RV function are also closely intertwined, the presence of RV dysfunction should also be recorded and included as a possible confounding factor for future analysis [8, 17]. Furthermore, we purport that measures or surrogates of the pulmonary vasculature should be considered as endpoints in future antifibrotic studies. While it is not practical or feasible to have baseline and follow-up RHCs in all patients, candidate surrogate measures include centrally adjudicated baseline and follow-up echocardiograms as well as baseline and follow-up NT-proBNPs.