A new mouse study reveals that carefully selected probiotics can lower blood sugar, body fat, and cholesterol levels while reshaping gut metabolism, suggesting a promising strategy for future diabetes prevention.
Study: Precision Probiotics Regulate Blood Glucose, Cholesterol, Body Fat Percentage, and Weight Under Eight-Week High-Fat Diet. Image Credit: Anusorn Nakdee / Shutterstock
In a recent study published in the journal Metabolites, researchers developed a precision probiotic cocktail to improve blood glucose homeostasis. Hyperglycemia is approaching epidemic prevalence worldwide, with about a third of the United States population estimated to have poor glucose homeostasis. Chronic hyperglycemia may lead to life-threatening conditions, such as neuropathy, cancer, nephropathy, retinopathy, diabetic ketoacidosis, and cardiovascular disease. It is also associated with metabolic syndrome, obesity, and reductions in quality of life and lifespan.
Probiotics are promising interventions for complications associated with hyperglycemia. A recent meta-analysis found significant improvements in the homeostatic model assessment of insulin resistance, high-density lipoprotein (HDL) cholesterol, and glycated hemoglobin with probiotic supplementation. However, there is a need for strain-specific probiotic combinations selected based on their high glucose-consumption capacity and optimized for glucose lowering.
The study and findings
In the present study, researchers developed a precision probiotic cocktail to enhance gut microbial glucose consumption, thereby improving weight loss and blood glucose control. Twelve gut microbial strains were tested for *in vitro* glucose consumption. These included Lactobacillus acidophilus, L. casei, L. gasseri, L. rhamnosus, L. plantarum, L. paracasei, L. salivarius, L. reuteri, Bifidobacterium bifidum, B. longum, B. animalis, and Nissle 1917.
A glucometer was used to measure glucose consumption by each strain after 24 hours of incubation in the Gifu anaerobic medium (GAM) and de Man, Rogosa, and Sharpe (MRS) broths. Glucose depletion in the MRS broth was significantly higher for samples inoculated with B. bifidum and Lactobacillus strains than for the uninoculated blank, L. salivarius, L. rhamnosus, L. gasseri, and L. reuteri consumed nearly all available glucose.
Glucose depletion in the GAM was significantly greater in all inoculated samples compared to the blank. Consistently, L. rhamnosus, L. salivarius, and L. reuteri showed the highest glucose depletion. Building on these results, six male C57BL/6J mice per group were fed a high-fat diet (HFD) for eight weeks, and L. rhamnosus, L. salivarius, and L. reuteri were selected for *in vivo* testing as a precision probiotic cocktail. Mice were gavaged with 5 × 10^8 CFU/100 μL of each strain in phosphate-buffered saline every other day, or saline alone (controls).
From week 2 of HFD feeding onward, mice that received the probiotic cocktail consistently had lower fasting blood glucose (FBG) levels than controls. FBG was measured weekly after a 6-hour fast using a handheld glucometer. Notably, controls showed significantly higher FBG at week 8 compared to baseline. In addition, the probiotic group consistently had significantly lower body weights at weeks 1 and 8 than the control group. The magnitude of differences in weight gain between groups increased over weeks 3 and 8, indicating continued benefits.
While both groups demonstrated weight gain, controls had the most significant increase from baseline. After eight weeks, terminal adiposity was nearly one-third lower in the probiotic group than in controls. Body fat percentage was quantified at week 8 by EchoMRI. The probiotic group had approximately 50% lower circulating insulin levels than the control group. The probiotic cocktail also had significantly lower levels of low-density lipoprotein (LDL) and very low-density lipoprotein (VLDL), total cholesterol (TC), and triglycerides compared to the controls.
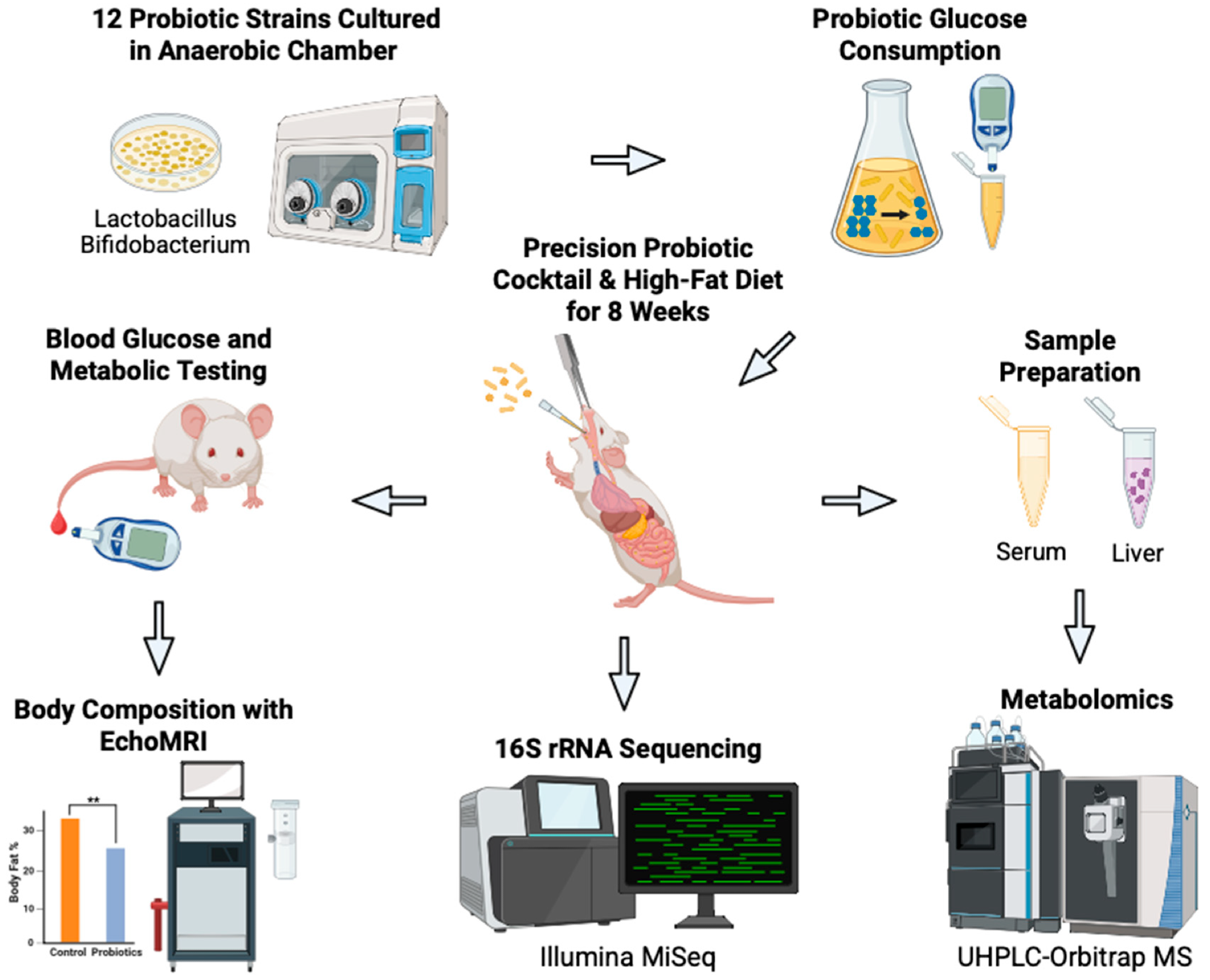
Schematic overview of our novel approach to develop precision probiotics for blood glucose control. Bacterial strains (n = 12) were tested in vitro to identify strains with the greatest glucose consumption capabilities. The top three strains were combined to produce the probiotic cocktail. The efficacy of the cocktail was then tested in vivo on C57BL/6J male mice on a high-fat diet who received oral gavages of the probiotics (n = 6) or vehicle PBS (n = 6) every other day for eight weeks. Body weight and blood glucose concentration were measured weekly. Terminal body composition was measured at eight weeks. Terminal serum and liver samples were harvested for metabolomic analysis. Fecal samples were collected for 16S rRNA sequencing and analysis. Created in BioRender. Patterson, J. (2025). Accessed on 3 July 2025. https://BioRender.com/bjkw2oe.
HDL levels were not significantly different between groups, although the TC/HDL ratio was significantly lower in the probiotic group. Further, terminal fasting serum samples were subject to untargeted metabolomics. This revealed significant differences in the concentrations of 62 metabolites between groups.
These included metabolites associated with adenosine triphosphate formation (D-ribose-1-phosphate), vitamin C metabolism products (L-threonic acid), energy-producing substrates (D-mannose, pyruvic acid, and D-glucose), and carnitine derivatives (O-propanoylcarnitine, 3-dehydrocarnitine, and 2-ethylacryloylcarnitine). A metabolic pathway analysis of serum samples from both groups revealed various energy production pathways.
Furthermore, liver samples were collected to assess the effects on tissue metabolites. L-threonic acid and oxoglutaric acid (an intermediate in the tricarboxylic acid cycle) exhibited increased liver abundance in the probiotic group relative to controls. In addition, L-lysine, aminoadipic acid, N6-acetyl-L-lysine, and methylmalonic acid were significantly elevated in the probiotic group, while creatinine was reduced.
The liver metabolic pathway analysis also identified various amino acid metabolism and energy production pathways. Finally, terminal fecal samples were subject to 16S rRNA sequencing. Faith’s phylogenetic α-diversity quantifications indicated significant changes in the probiotic group. There was a substantial upregulation of Muribaculaceae and Lachnospiraceae bacterium 609-strain and a decrease in Odoribacter in the probiotic group.
Conclusions
The study presented a precision probiotic cocktail that significantly reduced elevations in body weight and FBG in HFD-fed mice compared to controls. The cocktail significantly improved circulating insulin, VLDL/LDL, and TC levels. Metabolomic analyses revealed host metabolic adaptations for energy-producing pathways.
Findings are preclinical in a high-fat-diet mouse model with small, male-only cohorts; human efficacy requires larger clinical trials. Overall, the findings highlight the potential of precision probiotics in enhancing gut microbial glucose consumption and reducing glucose availability to the host.
Two authors are employed by MetaBiotics LLC, and one by Theriome Inc. Data are available upon request due to a filed patent. No external funding was reported.
Journal reference:
- Chi J, Patterson JS, Li L, et al. (2025). Precision Probiotics Regulate Blood Glucose, Cholesterol, Body Fat Percentage, and Weight Under Eight-Week High-Fat Diet. Metabolites, 15(10), 642. doi:10.3390/metabo15100642, https://www.mdpi.com/2218-1989/15/10/642