Study design
This was a hospital-based, comparative, cross-sectional analytical study.
Study site
The study was carried out at the paediatric cardiology clinic of the University of Nigeria Teaching Hospital, Enugu, Nigeria, a tertiary care hospital, and one of the national designated centres for cardiac excellence. The paediatric cardiology clinic provides specialized care for children with cardiac diseases and serves as a major referral centre from the south-east and south-south regions of Nigeria. The clinic treats approximately 76 patients with CCHD per year.
Study population
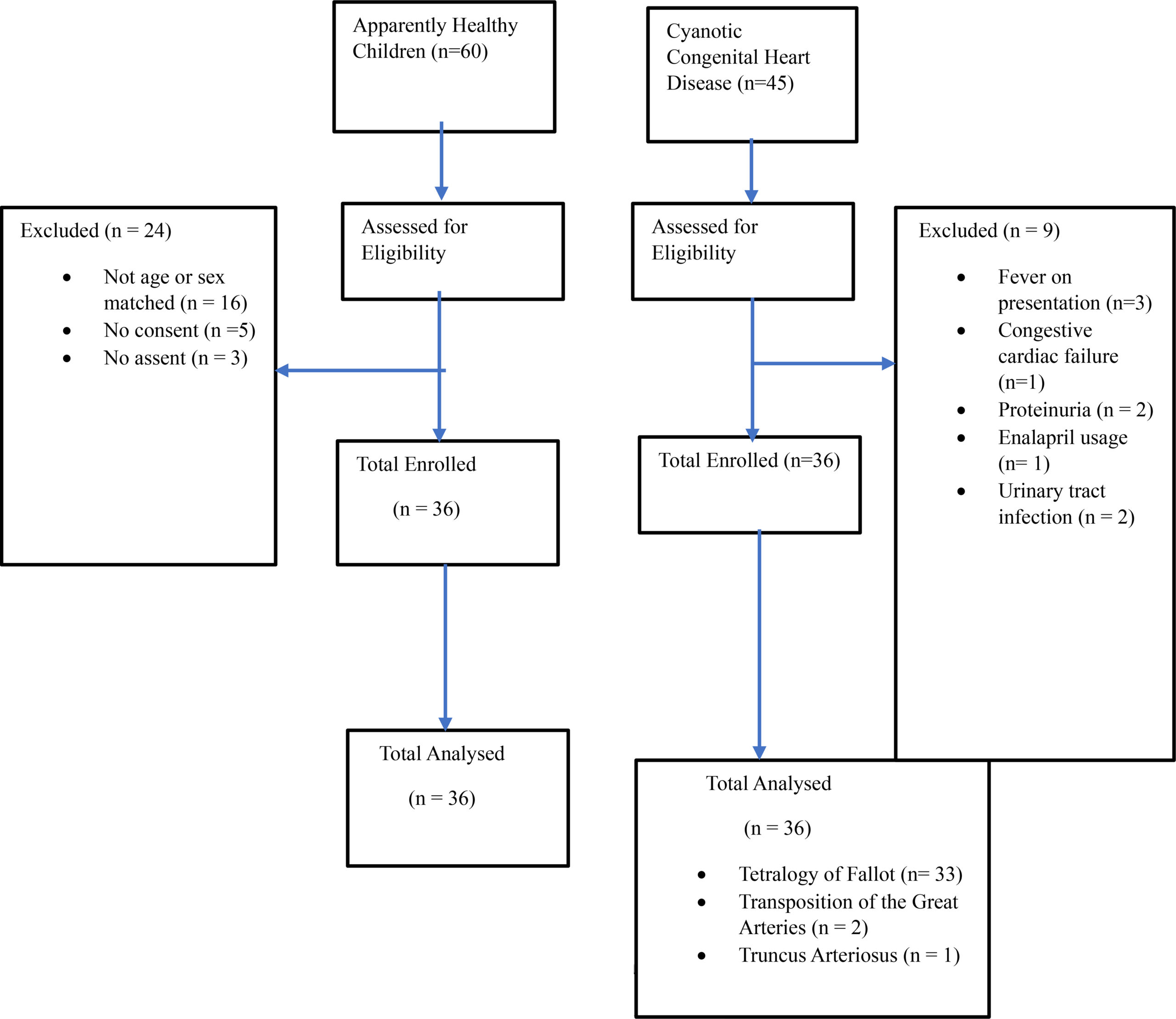
The subjects for the study were children aged one month to seventeen years, who had an echocardiographic diagnosis of CCHD and were attending the paediatric cardiology clinic of UNTH, whereas age- and sex-matched apparently healthy children who had recovered from minor illnesses such as uncomplicated malaria, gastroenteritis and uncomplicated respiratory tract infections and who were on follow-up at the children outpatient clinic of the same hospital, served as the comparative group. Children with corrected cyanotic congenital disease, congestive cardiac failure, urinary tract infection, chronic kidney disease (eGFR < 60 ml/min/1.73m2), and comorbidities such as sickle cell anaemia and diabetes mellitus were excluded from the study. In addition, those who were taking nephrotoxic drugs and who had febrile illnesses and proteinuria at the time of enrolment were excluded.
Sample size estimation
The sample size for the study was calculated using the formula for comparing two proportions [23]. The sample size was calculated using a power of 80%, a 5% level of significance, a sample proportion of group 1 of 28%10 and a sample proportion of group 2 of 4%10, after which a minimum sample size of 33 was obtained. A 10% non-response rate was thereafter applied to account for the possibility of missing data, misplaced samples, incomplete data, etc., resulting in a sample size of 37.
Patient enrolment
Subjects
All children presenting to the paediatric cardiology clinic had the essence of the study and the study procedure explained to them and their caregivers, and informed consent to participate in the study was obtained. Assent was also obtained from children aged seven years and above. To determine eligibility for the study, the children and their caregivers were interviewed, and thereafter, a case record form was filled out with information retrieved from the case file by the researcher. The information that was retrieved included type of congenital heart disease (based on echocardiographic findings), presence of comorbidities, past medical history to ascertain if corrective surgery had been performed, drug history, etc. For children who were found to have cyanotic congenital heart disease, historical information, such as the presence or absence of fever, urinary frequency, urgency, reduction in urine output, etc., was sought from the children and their caregivers by the researcher. Thereafter, a detailed physical examination, including measurements of the axillary temperature, oxygen saturation, blood pressure and height/length, as well as the exclusion of the presence of congestive cardiac failure, was performed on these children by the researcher.
After physical examination had been completed, the researcher then performed dipstick urinalysis on a spot urine sample produced by the children who had met all the entry criteria until this point. Children who tested positive for protein, glucose, leucocyte, and nitrite were excluded from the study and were counselled and referred to the nephrology clinic for further evaluation, treatment, and monitoring. Those who had negative or trace proteinuria then had the remainder of their urine samples immediately stored in the sample transport box for further testing for microalbuminuria in the laboratory. Blood samples were also collected from the same children who had negative or trace proteinuria, for haematocrit estimation and for serum creatinine measurement to exclude chronic kidney disease. Finally, after the estimated glomerular filtration rate (eGFR) was calculated, children whose eGFRs were ≥ 60 mls/min/1.73 m2 were consecutively enrolled into the study until the desired sample size was reached, after which the remainder of their urine samples were analysed for microalbuminuria in the laboratory.
Comparative group
The procedure for obtaining informed consent and assent for the comparative group was the same as that for the subjects. Children who were matched for age and sex with the subjects were interviewed together with their caregivers, and thereafter, a questionnaire regarding the presence of symptoms such as fever, urinary frequency and urgency, cough, fast breathing, difficulty in breathing, drug history, etc., was administered to them and/or their caregivers by the researcher. Thereafter, a detailed physical examination was performed on the comparative group, as was done on the subjects, and the same procedure for collection of urine and blood samples from the subjects was also performed on the comparative group. Those who met the inclusion criteria were subsequently enrolled into the study until the desired sample size was reached.
Sampling technique
Study participants who met the inclusion criteria were consecutively enrolled into the study until the sample size was reached.
Ethical considerations
Informed consent to participate in the study was obtained from the caregivers of all the study participants. Assent was also obtained from children aged 7 years and above. The study protocol was reviewed and approved by the Health Research and Ethics Committee of the University of Nigeria Teaching Hospital, Enugu, Nigeria, with reference number NHREC/05/01/2008B-FWA00002458-IRB00002323.
Clinical procedures
Oxygen saturation
All the participants were placed in a relaxed position; younger children sat on the caregiver’s thighs, whereas older children sat comfortably on a chair. Oxygen saturation was then determined by the researcher with a pulse oximeter (Fabrication Enterprises, 12-1927, Beijing). The oxygen saturation value displayed as soon as a good pulse was detected was recorded as the oxygen saturation of each participant.
Urine sample collection and Urinalysis
Urine samples were collected from the study participants between 8 am and 12 pm each day in properly labelled universal containers. Dipstick urinalysis was immediately performed on the urine samples using Combi 11 strips (Mission Expert U034-111, ACON, USA) by the researcher. The reagent strips were dipped and completely immersed in the urine sample for 2–3 s. The test pad colours for protein, glucose and nitrite were compared with the colour chart on the bottle containing the strips after one minute, whereas the test pad colour for leucocytes was compared with the colour chart on the bottle after two minutes for all the urine samples. Participants who tested positive for protein, glucose, leucocyte, and nitrite were excluded from the study, counselled, and referred to the paediatric nephrology clinic. Those who had negative or trace proteinuria had the remainder of their urine samples analysed for albumin/creatinine ratio in the laboratory.
Laboratory procedures
Haematocrit estimation
Haematocrit was estimated using a micro haematocrit centrifuge (KHT-400, Ocean Med+ England) by the researcher. This test is based on the principle that when anticoagulated whole blood is centrifuged, the space occupied by the packed red blood cells is defined as the haematocrit and is expressed as the percentage of red blood cells in a volume of whole blood [24]. The haematocrit values were read with a micro haematocrit reader (Hawksley, England), by the researcher.
Measurement of microalbuminuria
Urine creatinine determination
Urine creatinine was quantitatively determined using the alkaline picrate colorimetric method (Jaffe principle). (Randox Laboratories Ltd, CR8022, United Kingdom). The test is based on the principle that creatinine in alkaline solution reacts with picric acid to form a coloured complex. The amount of the complex formed, is directly proportional to the creatinine concentration of the urine [25].
Urine albumin determination
Urine albumin was quantitatively determined using the immunoturbidimetric method (Randox Laboratories Ltd. MA3828, United Kingdom). The test is based on the principle that the absorbance (340 nm) of the turbid solution resulting from the mixture of a urine sample and a buffer that contains an antibody specific for human albumin, is proportional to the concentration of albumin in the urine sample [26].
Urine albumin/creatinine ratio
The ratios of the values obtained for urine albumin and urine creatinine were expressed in milligrams (mg) of albumin per gram (g) of creatinine for each participant. Ratios in the range of 30–300 mg/g were defined as positive for microalbuminuria.
Statistical analysis
Data entry and analysis were performed using the International Business Machine – Statistical Package for Social Sciences (IBM-SPSS) version 21 (Armonk, New York, USA). The normality of the distribution of continuous variables was assessed using the Kolmogorov‒Smirnov test. Categorical variables were described using frequency counts and percentages.
The receiver operating characteristic (ROC) curve was used to categorize oxygen saturation and haematocrit into two groups at the most sensitive and specific levels for microalbuminuria (88.5% and 56%, respectively), and thereafter, the associations between microalbuminuria and age, oxygen saturation and haematocrit were tested with Fisher’s exact test and the Chi-square test.
Variables that were found to be statistically significant after the bivariate analysis were then added into the regression equation, for multivariate logistic regression, to determine the strength of the association. All tests of significance were two-tailed at 95% confidence interval, and the probability (p) value was considered significant, if the value was less than 0.05.