We enrolled 90 MAFLD patients (50 diabetic and 40 non-diabetic) as well as 40 healthy controls to assess the utility of PDGFRβ as a non-invasive biomarker for prediction of liver fibrosis in diabetic MAFLD patients. Most MAFLD patients in our study were females, and MAFLD subjects with T2DM were older than the nondiabetics as reported previously [31,32,33]. The waist circumference, waist/hip circumference ratio, and BMI were significantly higher in MAFLD subgroups than controls, as previously confirmed [34,35,36,37,38,39]. Similarly, visceral fat, fat percentage, and fat mass values were significantly higher in MAFLD subgroups compared to controls, in line with several previous studies [40,41,42,43,44]. Also, visceral fat as well as metabolic syndrome features were significantly higher in diabetic compared to nondiabetic MAFLD patients, in agreement with previous reports [45, 46].
PDGFRβ in our study was significantly higher in both MAFLD subgroups compared to controls. It was reported that PDGFRβ expression is extremely decreased in the healthy liver but markedly increased during liver injury [47]. We found significantly higher AST and ALT levels in MAFLD subgroups than controls, as previously reported [48, 49]. Serum albumin was significantly lower in diabetic MAFLD patients than controls, which can be due to the effect of T2DM on the kidney [50].
MAFLD subgroups had significantly higher total cholesterol, LDL, and triglycerides than controls. Also, lower HDL was observed in MAFLD subgroups compared to controls that reached statistical significance when comparing diabetic MAFLD and control groups. This pattern of dyslipidemia has been closely linked to MAFLD [51]. Moreover, diabetic MAFLD patients had significantly higher total cholesterol and LDL than the nondiabetic MAFLD group. Similar results were detected by previous studies [32, 52].
Liver steatosis assessed by MRI in our study was significantly higher in diabetic than non-diabetic MAFLD patients. A previous study also showed that hepatic steatosis was significantly higher in diabetic patients [53]. Diabetes is the most important risk factor for the development and progression of hepatic steatosis as well as liver fibrosis. Liver-related outcomes in patients with MAFLD are influenced by the advanced stage of fibrosis and not steatosis [54]. We assessed non-invasive scores of liver fibrosis in diabetic patients with MAFLD. AST/ALT ratio, APRI, King’s score, MAFLD fibrosis score, FIB-4, hepamet score, and PDGFRβ + FIB-4 were significantly higher in diabtetic MAFLD compared to non-diabetic patients. Drolz et al. concluded that non-invasive scoring systems are useful predictors of liver fibrosis in MAFLD subjects [55]. However, Bertot et al. found that non-invasive scoring systems are less accurate at predicting fibrosis progression in diabetic MAFLD patients [32]. Kings score is not yet validated in MAFLD subjects. It was initially evaluated for diagnosis of cirrhosis in patients with hepatitis C infection [25].
We also measured liver stiffness in MAFLD patients using MRE. Liver stiffness was significantly higher in diabetic than nondiabetic MAFLD patients. We found a significantly higher percentage of patients having significant liver fibrosis (≥ F2) in the diabetic MAFLD group compared to the nondiabetic group (40% vs. 17.5%). Park et al. found that the risk of significant fibrosis was higher in the diabetic MAFLD cohort than in the non-glucose-intolerant group [56].
Liver stiffness by MRE in our study correlated positively with APRI, King’s score, NAFLD fibrosis score, FIB-4, and Hepamet fibrosis score in total MAFLD patients. Fallatah et al. reported a significant positive association between liver stiffness measurements by fibroscan and AST/ALT ratio, APRI, and FIB-4 indices [57]. We also found a positive correlation between LSM and FIB-4 score in both diabetic and nondiabetic MAFLD patients, while APRI was positively correlated with LSM in nondiabetic MAFLD only. Similar results were reported by Shaji et al. [58]. Saran et al. found that the APRI score doesn’t correlate with imaging evidence of fibrosis in diabetic MAFLD subjects [59].
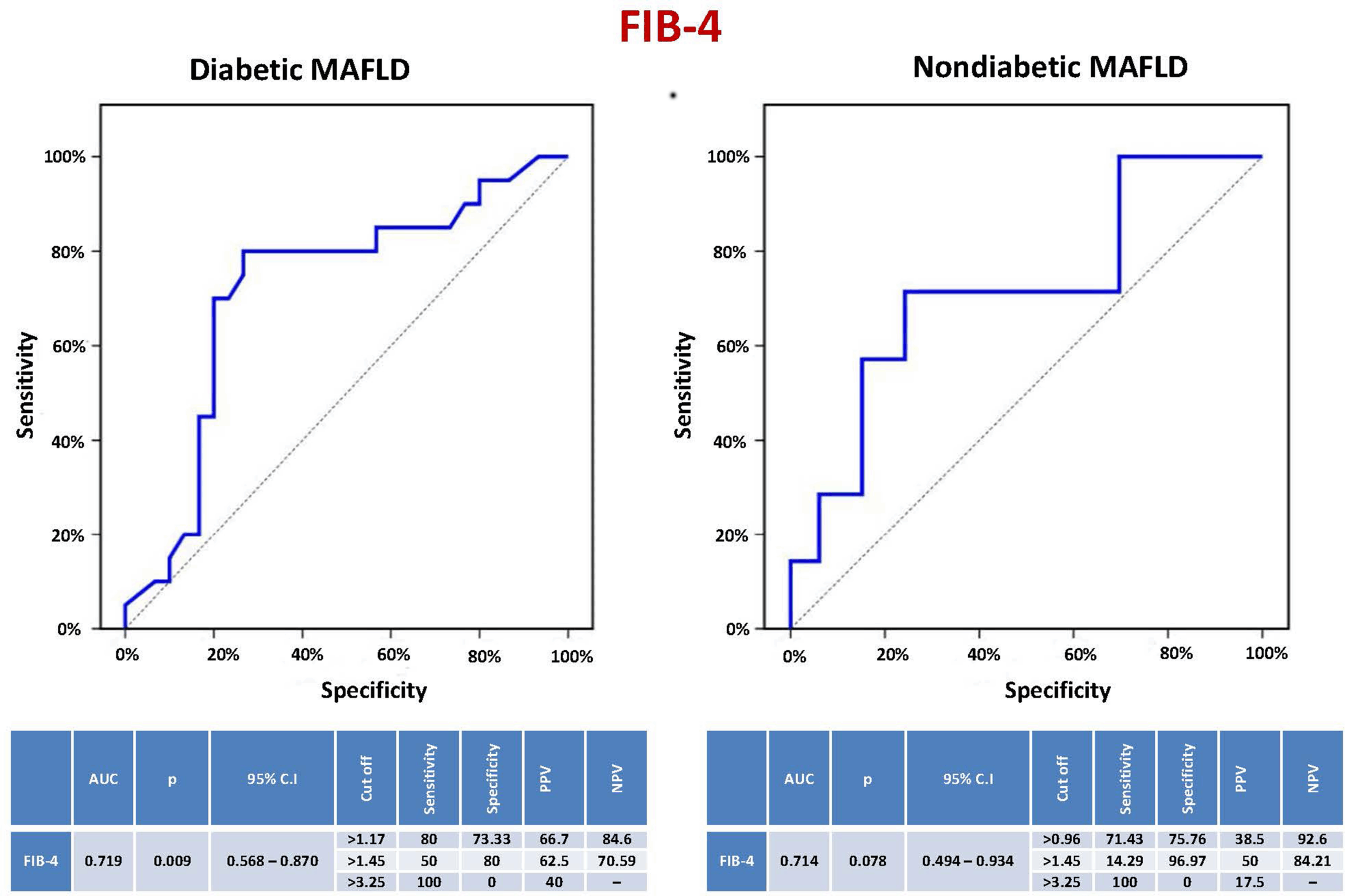
In our study, the sensitivity, specificity, PPV, and NPV of the FIB-4 score to predict significant liver fibrosis (≥ F2) in diabetic MAFLD patients were 80%, 73.33%, 66.7%, and 84.6%, respectively, at a cutoff level > 1.17 (AUC = 0.719, CI = 0.568–0.870, P = 0.009). In nondiabetics, they were 71.43%, 75.76%, 38.5%, and 92.6%, respectively, at a cutoff level > 0.96. Using previously validated cutoff levels of FIB-4 (1.45 and 3.25), used to predict advanced liver fibrosis (< F3) in viral hepatitis, in MAFLD patients to predict significant liver fibrosis (≥ F2) showed unsatisfactory performance. A meta-analysis of 36 studies revealed that the diagnostic accuracy of FIB-4 was relatively high in fibrosis stages ≥ F3 [60]. The most extensively validated non-invasive scores for determining a patient’s risk of advanced fibrosis in MAFLD are the FIB-4 score and the NAFLD fibrosis score. These tests are considered clinically valuable tools. Nevertheless, in at least 30% of the cases, these scores are suggestive of “indeterminate” ranges [61]. They have lower specificity in elderly patients, and new cutoff points have been suggested for those who are 65 years of age or older [62]. Moreover, these tests were not designed as screening tools but rather as part of a cohort study where severe fibrosis was more common. As a result, more specialized diagnostic tests must be performed in succession. Additionally, there might be variations in diagnostic performance between cohorts with and without diabetes, as well as possible racial disparities that could affect test accuracy [63].
We found that the performance of PDGFRβ as a non-invasive marker to predict significant liver fibrosis in diabetic MAFLD was superior to the FIB-4 score. The same finding was detected in a previous study by Lambrecht et al. that assessed serum PDGFRβ’s usefulness in predicting significant liver fibrosis across different etiologies (viral, alcoholic, and non-alcoholic liver disease in T2DM). They found that the predictive function of serum PDGFRβ is independent of disease etiology. Their study included 67 European MAFLD patients who were all diabetics [17]. Our study confirmed the diagnostic accuracy of serum PDGFRβ in Egyptian MAFLD patients in both diabetics and nondiabetics. A division of MAFLD subjects based on T2DM identified a strong discriminative ability of PDGFRβ for significant liver fibrosis. The performance of PDGFRβ was the best in diabetic MAFLD patients (AUC 0.907), with sensitivity, specificity, PPV, and NPV of 85%, 93.33%, 89.5%, and 90.3%, respectively, at a cutoff > 2.54, while its AUC in the Lambrecht et al. study was 0.6406. This may be due to differences in demographic criteria, number of studied populations, and liver stiffness assessment by acoustic radiation force impulse elastography [17].
PDGFRβ sensitivity, specificity, PPV, and NPV in non-diabetic MAFLD subjects were 85.71%, 51.52%, 27.3%, and 94.4%, respectively, at a cutoff value > 1.59, while the values were 57.14%, 87.88%, 50%, and 90.6% using the same cutoff (> 2.54) used in the diabetic MAFLD group. The sensitivity, specificity, PPV, and NPV of PDGFRβ + FIB-4 in diabetic MAFLD were all 100%, indicating that adding PDGFRβ to the FIB-4 score enhanced its ability to identify severe liver fibrosis in these subjects. Factors predicting significant liver fibrosis in the diabetic MAFLD group were PDGFRβ, smoking, visceral fat, and FIB-4 score, while PDGFRβ was the only independent predictor. Therefore, in diabetic patients with MAFLD, particularly individuals with FIB-4 intermediate ranges or elderly patients, the use of PDGFRβ or its incorporation with the FIB-4 score can improve significant fibrosis detection and prevent the need for an invasive liver biopsy or more costly imaging techniques. Similarly, serum PDGFRβ could complement or improve the performance of other non-invasive scores as well as non-invasive imaging modalities. PDGFRβ can also be useful in treatment monitoring of MAFLD patients since it is overexpressed during liver injury and its circulating levels can reveal information about the degree of liver fibrosis. Treatment options for the management of individuals with MAFLD can lead to resolution of hepatic fibrosis, during which hepatic stellate cells undergo apoptosis, become senescent, or revert to an inactive state with diminished PDGFRβ production [64].
Egypt has one of the highest prevalences of MAFLD at approximately 47.5%, with 56.7% having fibrosis. Given that the severity of fibrosis is the major determinant of both hepatic-related outcomes and mortality, patients with significant fibrosis need the closest monitoring. Identification of patients at risk is necessary for treatment decisions. The Egyptian guidelines for the diagnosis and management of MAFLD recommend using simple noninvasive biomarkers and scores of fibrosis for assessing disease severity and monitoring disease progression and treatment response. However, their cut-offs need to be further validated in Egyptian cohorts, and liver biopsy is still required in some cases, particularly in patients with indeterminant (gray) range scores [65]. Using PDGFRβ as a biomarker could improve non-invasive assessment of liver fibrosis in this patient population by increasing the accuracy of prediction and minimizing the gray zone.
The strengths of our study included that a strong case-control design was used with diabetic MAFLD patients, non-diabetic MAFLD patients, and healthy controls, enabling accurate comparison and validation of PDGFRβ as a biomarker. Also, the findings were validated using a variety of statistical methods, including multivariate logistic regression, increasing the dependability of the findings. Furthermore, this study distinguishes itself from previous studies on noninvasive markers for liver fibrosis by focusing solely on diabetic MAFLD patients. Previous studies have shown that noninvasive diagnostics intended for non-diabetic populations often work poorly in diabetic individuals. This work fills this gap by demonstrating that PDGFRβ more accurately predicts severe liver fibrosis in diabetic MAFLD patients, especially when combined with the FIB-4 score. Our findings have important clinical implications since they provide a more reliable non-invasive diagnostic tool for liver fibrosis in diabetic MAFLD patients, overcoming the limits of current biomarkers in these patients and reducing the need for invasive liver biopsies.
Nevertheless, our study has several limitations. A relatively small number of the studied subjects may impact the statistical power, and they were recruited from a single center and hence might not be truly representative of the broader population. Also, the study focused on PDGFRβ in comparison to only the FIB-4 score. A comparative analysis is still needed with other established biomarkers of liver fibrosis, such as alpha-smooth muscle actin (α-SMA), collagen, and integrins. Besides, we measured serum PDGFRβ at one timepoint, which may not accurately reflect the dynamic nature of fibrosis and its response to treatment. Additional patient monitoring is still needed to detect PDGFRβ correlation with long-term clinical outcomes. Conducting long-term, multi-center studies on a large population, including diabetic and nondiabetic subjects in a variety of liver fibrosis etiologies, along with cost-effectiveness analysis should be taken into consideration for subsequent research. The lack of a liver biopsy, which is regarded as the gold standard for determining the degree of disease activity and liver fibrosis, was another drawback of the current study. We used MRI and MRE to evaluate the liver steatosis degree and the liver stiffness, respectively, being more accurate to overcome the limitations of other non-invasive methods. A more conclusive evaluation of liver fibrosis would also be possible with the inclusion of a liver biopsy in future research.