Replacing F-18 sodium fluoride (NaF) PET/CT imaging with F-18 prostate-specific membrane antigen (PSMA) PET/CT can improve treatment decisions among men with newly diagnosed prostate cancer, researchers in Denmark have reported.
Among 160…
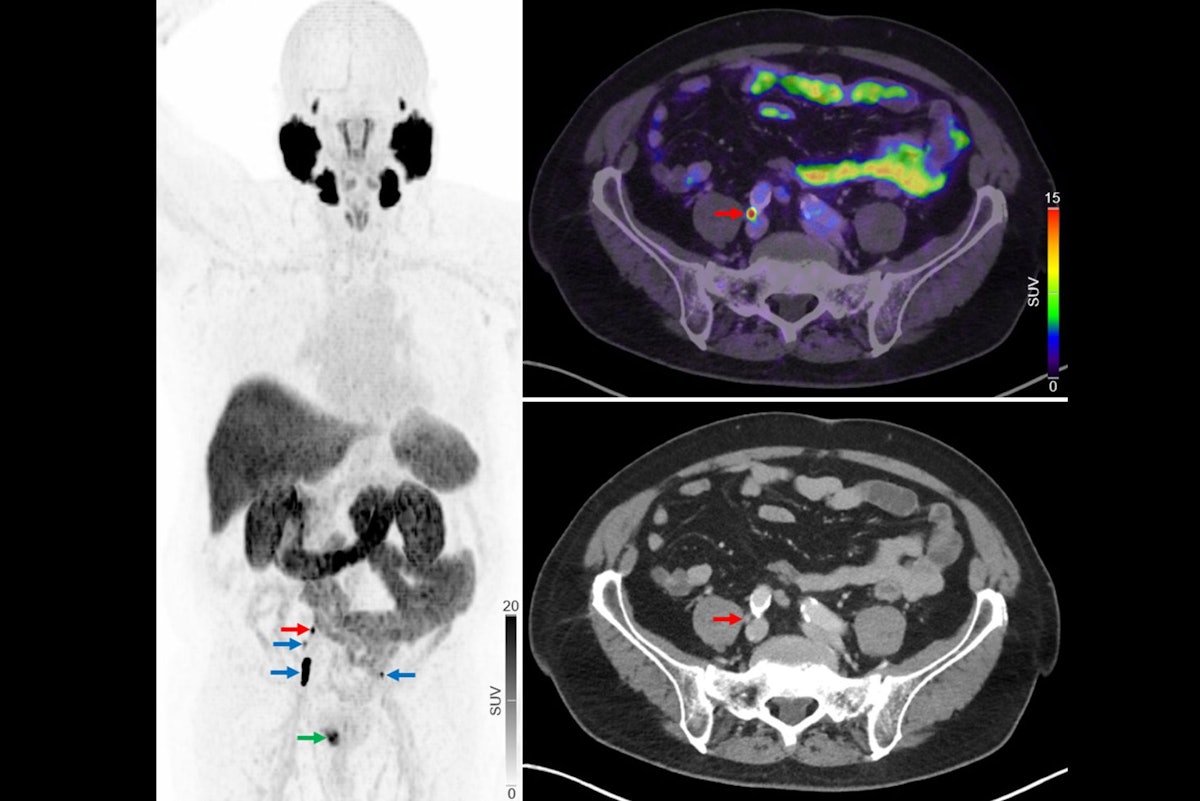
Replacing F-18 sodium fluoride (NaF) PET/CT imaging with F-18 prostate-specific membrane antigen (PSMA) PET/CT can improve treatment decisions among men with newly diagnosed prostate cancer, researchers in Denmark have reported.
Among 160…