Explanation for the choice of comparators {6b}
The study contains two arms including an experimental group and an active comparator group. Both groups perform standardized study exercises in a mechatronic device, reaching to outward targets displayed on a monitor. The set-up in the mechatronic device has been described in detail in a prior publication [20]. The active comparator group performs dosage-matched reaching exercises with the affected upper extremity that are identical to the experimental group except without the shoulder abduction loading component. Reaching with the arm on a support surface is critical to the study design to evaluate prescribed shoulder abduction loading as the key element in reaching exercise.
Intervention description {11a}
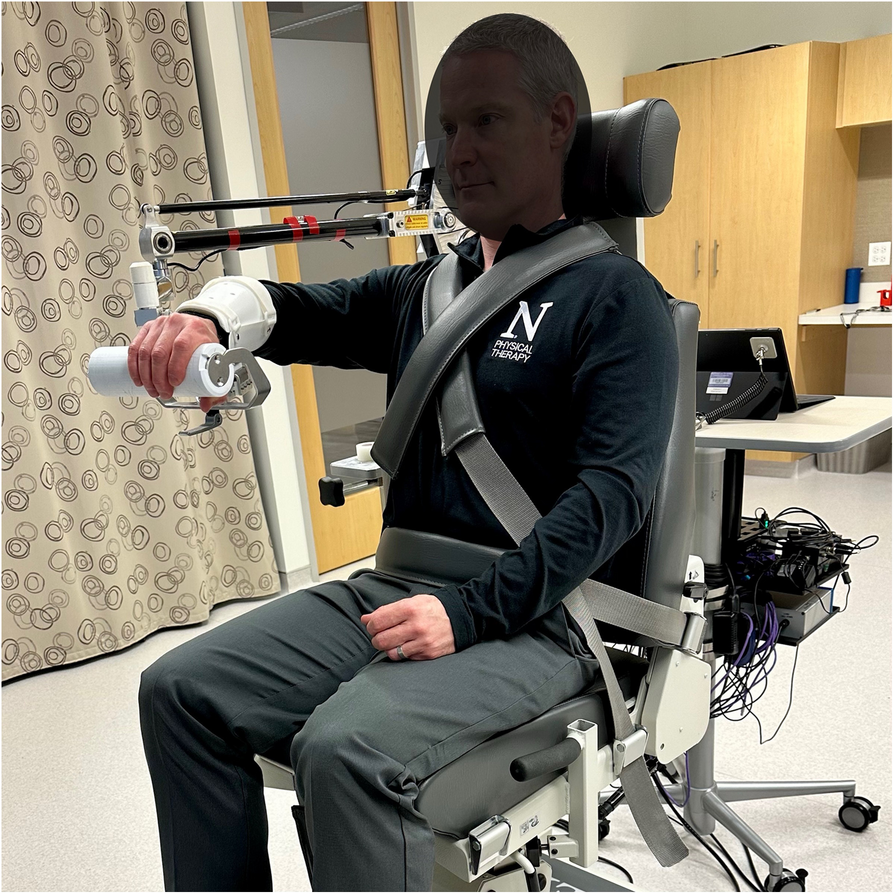
Set-Up: The study participant is seated in a Biodex chair and track system (Biodex Medical Systems, 49 Natcon Dr, Shirley, NY 11967, USA) with safety belts secured to limit trunk compensation (Fig. 1). The participant’s affected arm is coupled to a mechatronic device so that planar reaching exercises can be performed with the arm supported on the surface at shoulder height, either performed with or without shoulder abduction loading depending on the study group. The mechatronic device is instrumented, and upward/downward force is generated by a spring and pulley mechanism. This allows for precise prescription of abduction loading by the intervention therapist as a function of reaching kinematics performance.
Participant set-up in Biodex chair and mechatronic upper extremity device
Intervention
Active Comparator and Experimental Groups: Study intervention sessions are scheduled for 4 days per week during inpatient rehabilitation and 3 days per week during outpatient day-rehabilitation until discharge from the program. The intervention sessions are approximately 45 min in length and truncated at 1 h. Participants reach from a standardized start position to 4 targets placed at arm’s length. The targets are all within the forward workspace of the affected upper extremity. A maximum of 3 sets of 10 repetitions are completed to each target (maximum possible 120 repetitions within each intervention session). Rest periods of up to 10 s between trials and a fixed 1-min rest between sets are provided to avoid fatigue. Routine encouragement is provided by the intervention therapist to maintain consistent intensity and effort. The order of the sets is randomized via coded script in REDCap for each intervention session.
Active Comparator Group: The active comparator group performs horizontal planar reaching at shoulder height with the affected upper extremity toward outward targets while fully supported in the study equipment, like sliding along a frictionless table. Participants in the active comparator group move their arm along the frictionless horizontal surface and are not required to lift/abduct their arm during the reaching tasks.
Experimental Group: The experimental group intervention performs the same reaching exercises with the affected upper extremity toward outward targets in four directions, with the addition of shoulder abduction loading. This requires the participant to abduct the shoulder and maintain the arm a few inches above the horizontal plane during the reaching movement. Elevation of the arm above the horizontal plane is resisted or assisted by the study equipment with constant downward or upward force, respectively (abduction loading). The initial abduction loading level for the intervention is determined as a percentage of shoulder abduction strength that is quantified in the first evaluation session. Specifically, it is the amount of shoulder abduction force that permits reaching to at least 50%, but no more than 80%, of the distance to the outward target without the arm lowering to the horizontal surface. Study participants perform the reaching exercises at this abduction loading level until they reach greater than 80% of the distance to the target in at least 8 out of 10 repetitions for 2 out of 3 sets. The shoulder abduction load is then increased in intervals of 5 to 10% of abduction strength until the participants can again reach between 50 and 80% of the distance to the target. Shoulder abduction strength is reassessed weekly during the course of study treatment.
Criteria for discontinuing or modifying allocated interventions {11b}
Participants are required to receive daily intervention sessions except for weekends and days when evaluations are completed. There are no criteria for discontinuation or modification of interventions other than in the advent of an expected or unexpected adverse event (see adverse event reporting and harms).
Strategies to improve adherence to interventions {11c}
Attempts are made to accommodate participants’ scheduling preferences, encouragement is provided during intervention sessions, and rest breaks are provided as needed.
Relevant concomitant care permitted or prohibited during the trial {11d}
Participants are not prohibited from participating in concomitant care or research studies that do not involve an arm or hand intervention during the enrollment period.
Provisions for post-trial care {30}
In the event of injury or illness resulting from the research procedures, medical treatment is available during the inpatient phase of the study. Payment for this treatment is the responsibility of the participant. Information about research-related injuries is reported to the Office for the Protection of Research Subjects of Northwestern University.
Outcomes {12}
All outcomes are collected at baseline evaluation, weekly during inpatient rehabilitation, and every 2–3 weeks for those participants who attend outpatient day rehabilitation. After discharge from inpatient or outpatient day rehabilitation, assessments will continue every other month until 1 year post-stroke.
Primary outcome
Kinematic Evaluation: The primary outcome of the trial is change in the affected upper extremity reaching function, measured during a kinematic evaluation, by calculating the horizontal distance achieved during ballistic or maximum effort reaches to an outward target against gravity [21]. The testing set-up, reliability, and procedures have been described in detail in a prior manuscript [20]. This measure of efficacy precisely captures the functional reaching ability and, specifically, the impact of loss of independent joint control due to flexion synergy expression, the primary target of the study intervention. The outcome is measured at baseline, weekly (until discharge from inpatient rehabilitation, on average 4 to 10 weeks), and monthly evaluations (every-other month until 12 months post-stroke) to determine the change in outcome over the study period.
Secondary outcomes
Several secondary outcomes are included to capture changes in body function impairment, activity limitation, and participation restriction levels. Secondary outcomes are measured at all evaluation time points to determine the change in outcome over the study period. Shoulder abduction strength (Nm) and elbow extension strength (Nm) are measured isometrically with a force and torque sensor. Loss of independent joint control is measured during a kinematic evaluation by determining the maximum abduction load (N) achieved during a reach to two standardized targets (near and far). These two measures of efficacy represent the emergence and takeover thresholds of flexion synergy expression, defining the boundaries of loss of independent joint control, or in other words, the severity of flexion synergy motor impairment [21]. The testing set-up, reliability, and procedures have been described in detail in a prior manuscript. Three standardized clinical outcome measures including the arm motor Fugl-Meyer Assessment, the Action Research Arm Test, and the Stroke Impact Scale are administered [22].
Participant timeline {13}
All consented participants undergo screening for eligibility, which includes a motor evoked potential (MEP) assessment using transcranial magnetic stimulation (TMS, see description below), and a baseline evaluation. The MEP assessment is conducted using single pulse TMS (DuoMAG MP TMS stimulator, Deymed Diagnostic s.r.o. Kudrnacova 533, Hronov 549 31, Czech Republic) and is performed as an estimate of corticospinal tract integrity for the affected upper extremity. The presence or absence of either a resting or active MEP in the extensor carpi radialis or flexor digitorum indices muscles is determined using a previously described protocol [23]. If TMS is contraindicated, the participant continues in the study without the TMS assessment. After randomization, participants begin daily study interventions Monday–Friday for the duration of their hospital stay in parallel with inpatient rehabilitation standard of care. Study evaluations occur weekly during the inpatient phase of the study, and only one study activity (intervention or evaluation) occurs on a given day to avoid disruption to participants’ prescribed therapy schedule. If a participant is discharged to an outpatient day-rehabilitation center that is part of the study site, daily interventions continue, and evaluations occur every 2–3 weeks. If a participant does not attend outpatient day-rehabilitation, the intervention portion of the study is concluded, and follow-up evaluations are completed every other month until one year after the date of stroke onset. Figure 2 displays a schematic of study activities from enrolment through the final study evaluation.
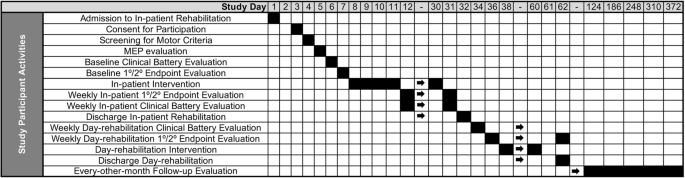
Schematic of the approximate timeline for participant study activities for the duration of the study period
Sample size {14}
The study targets an enrollment of 86 participants. A sample size of 72 is required to detect a change in the normalized reaching function of 0.1 in the experimental group compared to the active comparator group. This calculation is based on previous data [17] of individuals with chronic severe stroke where pre- and post-data have mean values of 0.32 and 0.31 for the control group and 0.25 and 0.34 for the experimental group, respectively, and the pre-post data have a correlation of r = 0.89. This data set is utilized for sample size estimation since the experimental and active comparator interventions are identical to the protocol in this study. Finally, assuming a 16% attrition from prior work, a sample size of 86 participants is estimated.
Recruitment {15}
Patients admitted to inpatient rehabilitation with a diagnosis of stroke are reviewed daily by authorized study team members for rapid identification of potential study participants, ideally within 48 h to optimize the study timeline. All individuals admitted with a diagnosis of stroke are counted and documented in deidentified format for CONSORT reporting. If a candidate is identified from chart review, study personnel communicate with the potential participant’s care team to gather further information regarding anticipated eligibility. A study team member then approaches the potential participant, discusses the study, and answers all questions in understandable language. If the individual is interested, the study team member reviews the consent form, and the potential participant has the opportunity to discuss the study with family prior to providing written consent.
After consent, an authorized study team member initiates screening. All consented participants undergo a screening assessment with the arm portion of the Chedoke McMaster Stroke Assessment (CMSA). The CMSA (primary motor inclusion criteria) is time efficient, reducing interference with inpatient therapy activities and quickly identifying individuals with severe impairment. Consented participants who fail to meet inclusion criteria are terminated from the study (if impairment is too minor, CMSA > 3). However, if the participant does not yet qualify due to a CMSA score of 1, they are followed during their inpatient episode of care until CMSA score increases to a 2 to facilitate recruitment. Enrollment for the study began on 8/27/2021 and will continue through 8/31/2025.