Want to speak at AMA: Energy 2025 or AMA: Automotive & Mobility 2025? Submit your application now!
Researchers at the University of British Columbia’s Okanagan (UBCO) campus have bioprinted a human airway model combining key lung cell types within a structure that mimics blood vessel function.
Led by Dr. Emmanuel Osei, Assistant Professor at Irving K. Barber Faculty of Science, the team used CELLINK’s LUMEN X bioprinter to construct a tri-culture system that offers a closer match to real lung tissue than traditional models.
Conventional lab systems often rely on flat cell cultures or animal models, both of which struggle to capture the complexity of human lungs. These approaches typically leave out the role of vasculature, limiting the ability to study how blood vessels interact with airway cells to regulate inflammation, immune responses, and tissue repair.
Published in Biotechnology and Bioengineering, the new model addresses this by introducing vascular channels lined with endothelial cells, combined with embedded lung fibroblasts and a surface layer of airway epithelial cells. Together, these elements create a more complete and responsive tissue environment.
“To conduct our research and the testing that’s required—where we’re studying the mechanisms of complex lung diseases to eventually find new drug targets—we need to be able to make models that are comparable to human tissues,” explained Dr. Osei.

Hydrogel blend supports cell survival
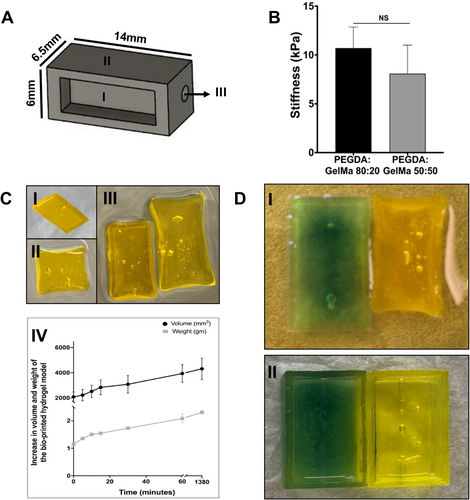
To build the structure, the researchers used a hydrogel blend of polyethylene glycol diacrylate (PEGDA) and gelatin methacrylate (GelMa) in an 80:20 ratio. This combination yielded a Young’s modulus of 10.7 kPa, within the physiological range of native lung tissue, and allowed the scaffold to expand by over 100% in both volume and weight upon hydration.
The material balance provided the right mix of stiffness and porosity to support cell survival while allowing fluids and signals to move through the tissue. Fibroblasts were printed directly into the gel, endothelial cells were seeded inside the vascular channels, and epithelial cells were added on top. The printing process was carefully optimized to maintain the shape of the scaffold and protect cell health throughout fabrication.
Imaging confirmed that each cell type was positioned correctly and maintained its expected form. Epithelial cells formed neat, barrier-like layers on the surface. Fibroblasts displayed rounded or spindle-shaped structures, depending on their location and the hydrogel properties. Endothelial cells created smooth, continuous linings inside the channels.
Cell viability remained high across the model, with survival rates of 93.8% for fibroblasts and 90.8% for epithelial cells in co-cultures, and slightly lower but still robust rates of around 87% for all three cell types in tri-cultures, noteworthy figures for bioprinted systems.
To test whether the model could replicate disease responses, the team exposed it to cigarette smoke extract. The tri-culture released higher levels of inflammatory markers such as interleukin-6 and interleukin-8, consistent with how real lung tissue reacts to smoke exposure. When compared to simpler co-culture models without vasculature, the tri-culture showed distinct immune behavior, reinforcing the importance of including blood vessels in realistic lung simulations.
The team also demonstrated that the vascular channels could support fluid flow using a peristaltic pump. This feature allowed for the simulation of blood movement through the tissue, adding another layer of realism. While some clumping of endothelial cells was observed during flow, the system overall functioned reliably and provided dynamic conditions not possible with static cultures.
“Our model is complex, but due to the reproducibility and optimal nature of bio-printing, it can be adapted to include additional cell types or patient-derived cells, making it a powerful tool for personalized medicine and disease modelling,” said Dr. Osei.

Efforts in 3D printed lung tissue engineering
Although fully functional bioprinted lungs remain under development, researchers are steadily advancing the creation of lung tissue models designed for studying diseases and testing new drugs.
Recently, McMaster University-spinout Tessella Biosciences developed a stretchable bioink that enables 3D printing of soft lung tissue capable of expanding and contracting like real lungs.
Unlike many existing bioinks that require cold environments and lose shape post-printing, Tessella’s formulation cures in under an hour and remains stable at body temperature. Compatible with standard lab printers, the material allows for more realistic in vitro models to study diseases like COPD and pulmonary fibrosis. The team is also exploring broader uses in regenerative medicine, including printable skin grafts and tissue patches.
Last year, biotechnology research company Frontier Bio announced the development of lab-grown lung tissue by integrating bioprinting with the natural self-organizing behavior of stem cells.
Using a custom mix of lung cells and biomaterials, the process guides cells to form intricate structures that replicate bronchioles, alveolar sacs, and cilia, mimicking the branching complexity and vital functions of real lung tissue, including mucus and surfactant production. Designed to address the limitations of animal models, this engineered tissue offers a more human-relevant platform for studying diseases like COPD and lung cancer, while also laying the groundwork for future transplant applications and broader regenerative therapies.
What 3D printing trends should you watch out for in 2025?
How is the future of 3D printing shaping up?
To stay up to date with the latest 3D printing news, don’t forget to subscribe to the 3D Printing Industry newsletter or follow us on Twitter, or like our page on Facebook.
While you’re here, why not subscribe to our Youtube channel? Featuring discussion, debriefs, video shorts, and webinar replays.