Daniel Day-Lewis recently told the U.K.’s Big Issue that Brian Cox is one of the reasons he’s been dragged into an unwanted debate around Method acting. Cox has spoken out against Method acting in various interviews over the last few years,…
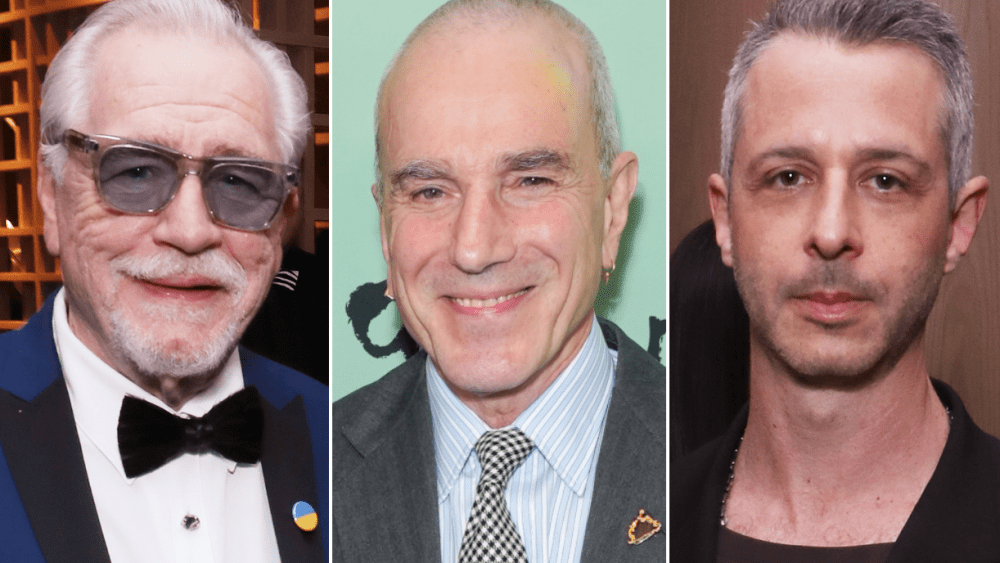
Daniel Day-Lewis recently told the U.K.’s Big Issue that Brian Cox is one of the reasons he’s been dragged into an unwanted debate around Method acting. Cox has spoken out against Method acting in various interviews over the last few years,…