S. boydii infections are uncommon, particularly in immunocompetent individuals. This case represents only the second reported instance of S. boydii detected via mNGS, emphasizing the utility of advanced molecular diagnostics in identifying slow-growing or fastidious pathogens. Typically, S.boydii colonies grow within 1–7 days; however, under the guidance of mNGS, incubation was extended for 20 days before colonies finally emerged; however. This case provides a detailed morphological atlas of S. boydii, including its dynamic growth characteristics on Sabouraud dextrose agar and blood agar over 20 and 31 days, as well as microscopic features of hyphae and conidia. The diagnostic-therapeutic process of this case was implemented as follows: mNGS detection → Microbial culture incubation extension (20days) →MALDI-TOF MS confirmation →Antifungal susceptibility-guided therapy (Fig. 3). mNGs findings can unveil cryptic pathogens like Scedosporium, Use these results totrigger intentional, extended targeted cultivation – challenging standard incubation timesespecially in non-immunocompromised hosts with underlying lung disease. Seamlessclinician-laboratory collaboration is paramount for success.
S. boydii diagnostic flow chart
Due to changes in the bacterial nomenclature, we conducted a search on PubMed and found only 8 case reports of infections caused by S. boydii from 2016 to 2025(Table 1) [6, 8, 10,11,12,13,14,15], with this article being the 9th. Among these, only one report used mNGS for strain identification [14], making this the second case. Most of the patients (6/8) were over 50 years old, and infections predominantly occur in individuals with compromised immune function. The infection sites were quite extensive, involving the brain, heart, lungs, and even bone marrow. Voriconazole was used as a first-line treatment, with half of the patients receiving this medication. The duration of strain culture varied from 1 to 7 days, and clinical identification typically combined morphological analysis with DNA sequencing.
S.boydii infections frequently cause invasive fungal infections in immunocompromised patients, include post-transplant status, trauma, postoperative surgery, history of corticosteroid use, near-drowning, and idiopathic autoimmune diseases [6]. Scedosporium infections can affect any organ, with common sites being the lungs (27%), skin (23%), eyes (8%), and central nervous system (13%) [9]. Pulmonary infections caused by Scedosporium species most commonly present with cough (60.0%), followed by hemoptysis or blood-streaked sputum (50.0%) and fever (35.0%). When microbiological confirmation is unavailable but imaging findings strongly suggest fungal infection, management strategies should be adjusted based on host factors and radiological features. Characteristic CT findings of Scedosporium infections include consolidation, nodules, infiltrates, cavitary lesions with surrounding infiltrates, necrotizing pneumonia, abscesses, and pleural effusion [16, 17]. A small proportion of patients may develop fungal balls [18]. Imaging findings in Scedosporium infections lack specificity, making accurate etiological diagnosis particularly important.
Among the detection methods, clinical microbial culture has the advantage of being the gold standard for accurate strain identification and being cost-effective. However, its disadvantages include being time-consuming, potentially delaying diagnosis, and being prone to false negatives/misidentification. Species confirmation can be achieved through ITS and β-tubulin gene sequencing or other molecular methods [19]. MALDI-TOF MS offers the advantage of being economical and efficient, enabling species-level identification within minutes. However, its limitations include the requirement for prior pathogen isolation and culture, as well as insufficient database coverage for rare species, leading to reduced identification accuracy. mNGS has the advantage of detecting pathogens directly from clinical samples without the need for culture, enabling broad-spectrum detection of bacteria, fungi, viruses, and parasites. It provides results relatively quickly, typically within 24–48 h. However, its disadvantages include high cost, difficulty in distinguishing infection from colonization, and potential false positives due to environmental or human DNA contamination.
Infection with S. boydii is clinically rare and nonspecific, making early diagnosis particularly challenging. Currently, most diagnoses rely on molecular methods [19]. In this case, after S. boydii was detected in BALF by mNGS, the laboratory staff intentionally paid attention to extending the cultivation time. However, it took up to 20 days for colony growth. The possible reasons may be the difference in metabolic rate between different species and different isolates of Scedosporium, and poor culture conditions: temperature, humidity or oxygen partial pressure deviating from the optimal range may inhibit growth. It is worth noting that S. boydii had a higher initial positive rate in the first generation culture under 37℃5% CO2 environment. Under typical conditions, Scedosporium spp colonies grew within 3–7 days at 37 °C on SDA medium, reaching a diameter of 60–70 mm after 10 days [7, 19]. Unlike what was described in the relevent literature, the strain in this case grew extremely slowly and did not grew until 20 days later. It can be said that the fungus might not have been cultivated if the culture time was not deliberately extended. This case once again highlights that compared to microbial culture, mNGS offers broader pathogen coverage, higher detection rates, improved accuracy, and shorter turnaround times, which are particularly advantageous for detecting unknown species, slow-growing, or intractable pathogens [14, 20]. Later, the fungus was cultured and drug sensitivity results were obtained. This case is a perfect combination of mNGS with traditional cultivation techniques and MALDI-TOF MS pathogen identification technology.
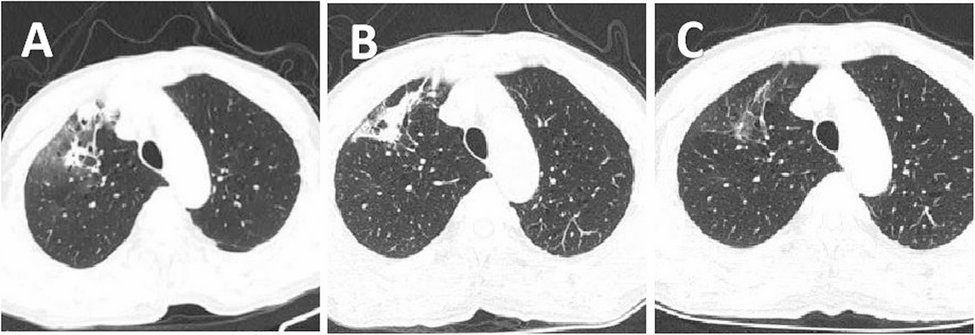
The initial antimicrobial treatment of the patient was unsatisfactory mNGS indicated that S. boydii might be the causative pathogen. The patient’s past medical history was questioned and it was found that the patient had been exposed to a musty basement environment in the past six months and had a history of COPD. Scedosporium species can colonize the lungs of individuals with chronic lung disease [15]. 20 days later, the strain isolated from BALF was identified as S. boydii, which was consistent with mNGS, further confirming that the pathogen was S. boydii. After six weeks of treatment with voriconazole, the patient’s clinical symptoms, chest CT results, and laboratory examination showed significant improvement, indicating the effectiveness of the antifungal therapy.
Based on current case reports and treatment guidelines, voriconazole monotherapy is the first-line treatment for Scedosporium infections, with surgical debridement recommended if necessary to reduce pathogen burden reduction [21]. Although the epidemiological breakpoint for Scedosporium has not yet been established, an MIC value of voriconazole below 2 µg/mL may suggest a favorable treatment outcome [8]. Isavuconazole, posaconazole, or itraconazole monotherapy may be used as second-line treatment [22]. If Scedosporiosis progresses or shows poor response to initial antifungal therapy, guidelines recommend either posaconazole or voriconazole alone, or a combination of voriconazole or echinocandin [22, 23]. In immunocompromised hosts, pathogen clearance efficacy is highly dependent on systemic immune reconstitution and management of underlying comorbidities. The study by Neoh et al. indicated that most infections caused by Scedosporium species responded to antifungal therapy during a 3-to-6-month follow-up period, suggesting that extended treatment for at least 6 months may be required.