HTML
Background and Establishment of World Hepatitis Day
Viral hepatitis remains a major global public health threat. Five principal types of hepatitis viruses — designated A, B, C, D, and E — each cause liver disease but differ significantly in transmission routes, clinical severity, and prevention strategies. Chronic infections from hepatitis B and C viruses represent the leading causes of cirrhosis, hepatocellular carcinoma, and viral hepatitis-related mortality. According to World Health Organization (WHO) estimates (1), approximately 254 million people were living with chronic hepatitis B in 2022, with 50 million affected by hepatitis C (Figure 1). The estimated number of new viral hepatitis infections declined from 3 million in 2019 to 2.2 million in 2022 (2–3), including 1.2 million hepatitis B cases and nearly 1 million hepatitis C cases. By the end of 2022, nearly 7 million patients were receiving hepatitis B treatment, while 12.5 million had undergone hepatitis C therapy (2–3). Nevertheless, viral hepatitis still caused around 1.3 million deaths annually (Figure 1) (4), with low- and middle-income countries bearing the heaviest burden — accounting for over 85% of global hepatitis-related mortality.
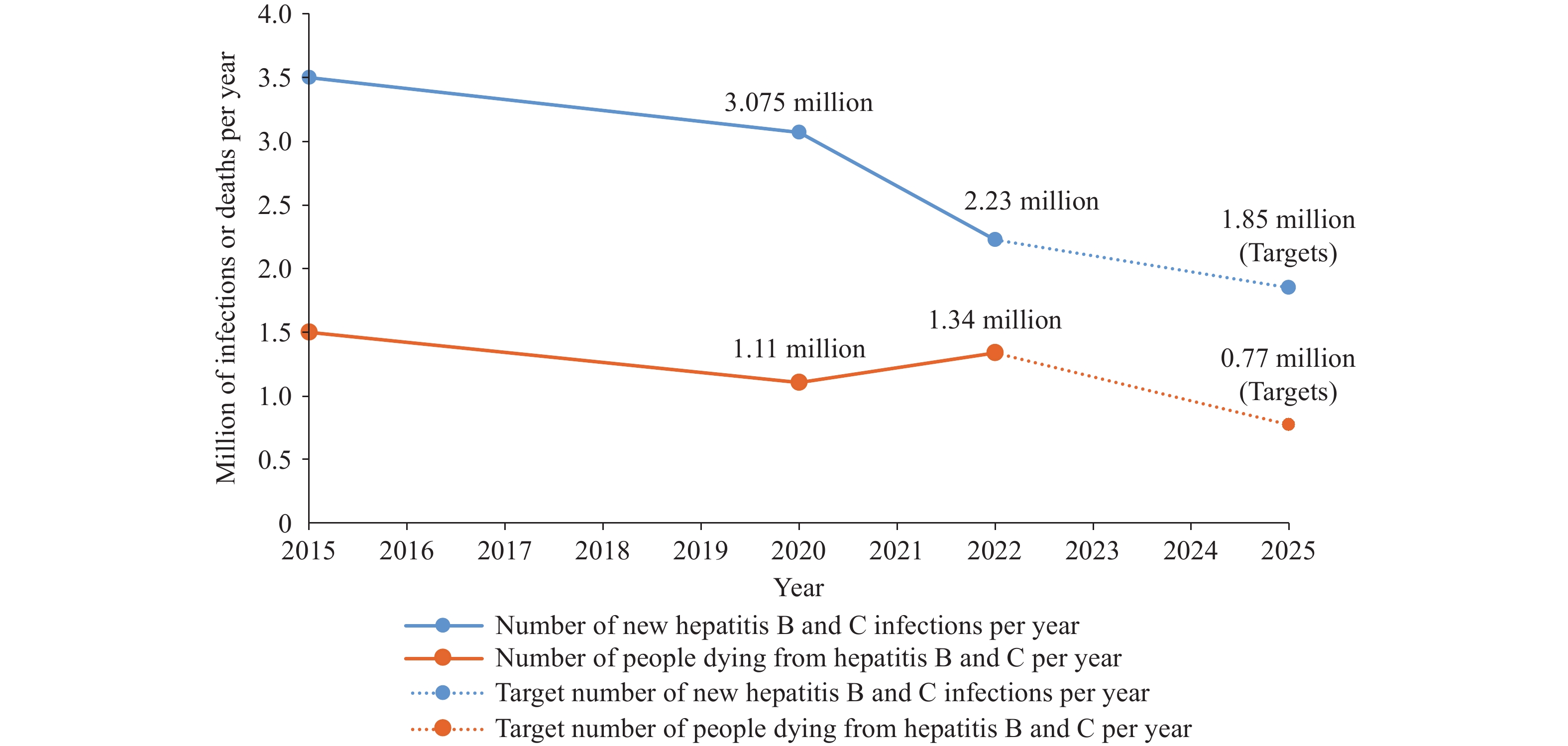
Trends in incidence and mortality of hepatitis B and C, 2015–2025.
July 28, 2025 marks the 15th anniversary of World Hepatitis Day. World Hepatitis Day was established through Resolution WHA63.18 at the 63rd World Health Assembly in 2010. July 28 was chosen to honor Dr. Baruch Blumberg’s birthday, the scientist who discovered the hepatitis B virus. The establishment of World Hepatitis Day aimed to unify regional hepatitis awareness campaigns under a single global observance. This decision carried profound significance: 1) Consolidating previously fragmented regional hepatitis awareness initiatives; 2) Creating a platform for coordinated global action; 3) Catalyzing the inclusion of hepatitis elimination in the Sustainable Development Goals (SDGs). Today, World Hepatitis Day stands as 1 of the most influential global public health advocacy campaigns, engaging over 100 countries annually. The theme for World Hepatitis Day 2025 is “Hepatitis: Let’s Break It Down”, which urges immediate measures to eliminate economic, societal, and structural obstacles — including stigma — that hinder the eradication of hepatitis and prevention of liver cancer.
Key Strategies and Activities of World Hepatitis Day Across Different Phases
Awareness Building Phase (2010–2015)
This initial phase concentrated on establishing foundational education initiatives and comprehensive awareness campaigns. The primary objective was to create a unified global advocacy framework that could effectively disseminate hepatitis knowledge through diverse communication channels. A pivotal achievement occurred in 2012 when WHO launched its inaugural Global Hepatitis Strategy, formally incorporating hepatitis prevention and control measures into the international health agenda.
Strategy Implementation Phase (2016–2021)
The 2016 Global Health Sector Strategy established ambitious 2030 elimination targets, catalyzing significant global progress. Key achievements during this period included: 1) Global three-dose hepatitis B vaccine coverage increased substantially from 82% to 87%; 2) Direct-acting antivirals (DAAs) demonstrated remarkable efficacy with cure rates exceeding 95% for hepatitis C; 3) Thirty countries developed comprehensive national elimination plans aligned with the 2030 goals of achieving a 90% reduction in new infections and 65% reduction in mortality (5).
Acceleration Phase (2022–2025)
The period following 2021 witnessed unprecedented momentum in global hepatitis elimination efforts (Figure 2), marked by several transformative breakthroughs: 1) WHO’s updated treatment guidelines in 2023 streamlined clinical protocols and enhanced treatment accessibility; 2) Egypt achieved a historic milestone by becoming the first country to receive WHO elimination certification in 2024; 3) Gavi’s expanded funding cycle launched in 2024 propelled global hepatitis B vaccine coverage beyond the critical 90% threshold by 2025 (6). The cumulative impact of these initiatives is reflected in treatment scale-up: between 2014 and 2023, an estimated 12,748,000 hepatitis C patients received DAAs treatment (7).
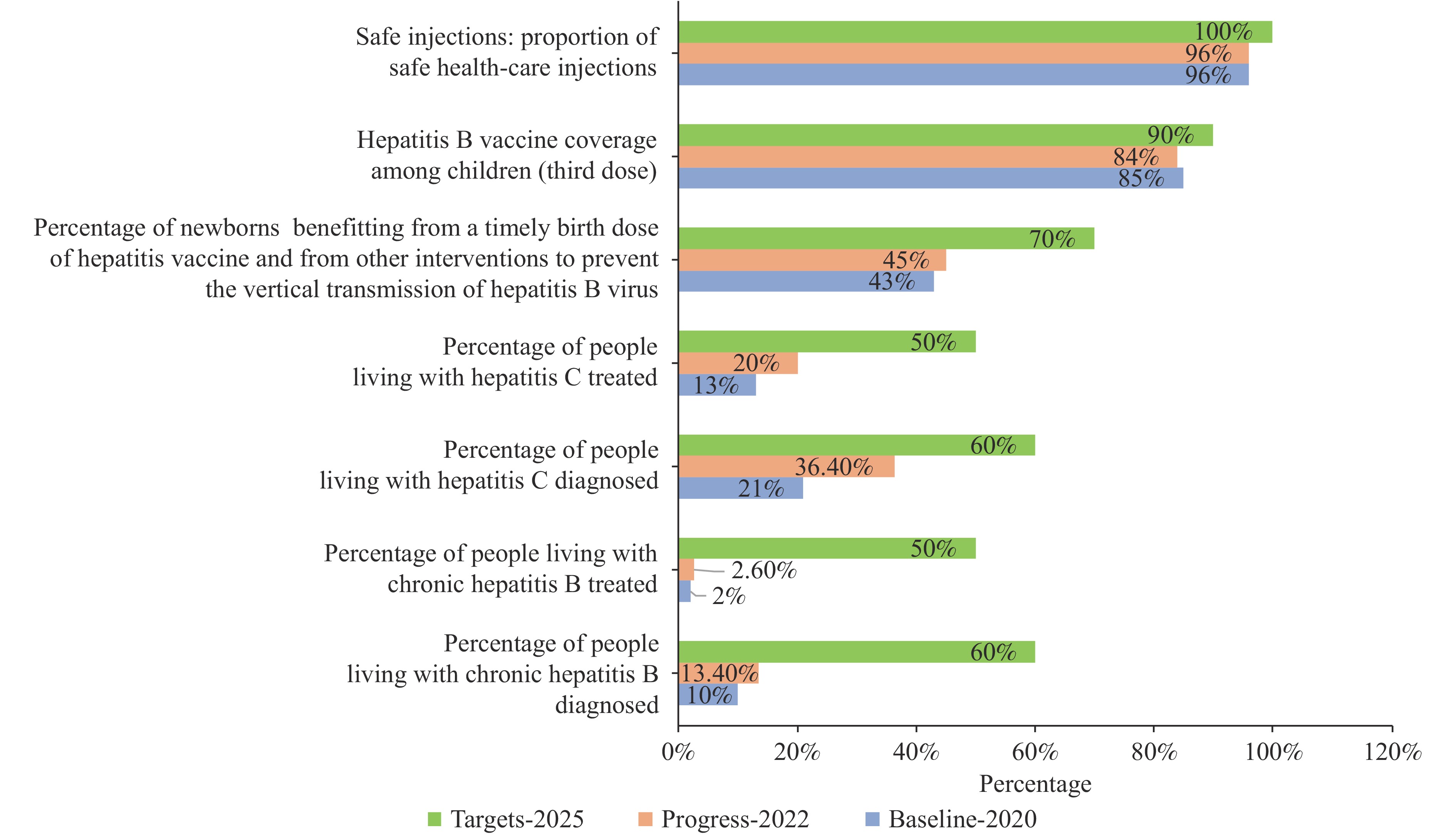
Progress towards global viral hepatitis targets.
Current Progress
Enhanced Prevention Efforts
Global hepatitis B surface antigen (HBsAg) prevalence among children under 5 years has achieved the 2025 target of ≤0.5% (2,8); universal coverage of safe injection practices has been attained (9); mother-to-child transmission prevention demonstrates success rates exceeding 97% (8).
Improved Treatment Accessibility
DAAs treatment costs have decreased to $75 per capita in low- and middle-income countries (4); novel hepatitis B therapeutics, including RNA interference (RNAi) therapies, are advancing through Phase III clinical trials; cumulative global hepatitis C treatments continue to increase annually. By 2025, the estimated cumulative number of individuals treated for hepatitis C worldwide reaches approximately 23–25 million.
Innovation and Scale-up of Diagnostic Technologies
High-sensitivity point-of-care testing (POCT) tools have gained widespread adoption, substantially improving screening efficiency in primary care settings. For instance, the enhanced sensitivity of HBsAg testing technologies has established this marker as a critical surveillance indicator for hepatitis B infection in pediatric populations. Multiple countries have implemented comprehensive national hepatitis registries and databases, integrating epidemiological surveillance data with treatment records. These systems facilitate real-time monitoring of elimination progress through validated mathematical models (e.g., the WHO framework), providing robust evidence for policy refinement and strategic adjustments.
Strengthened Policy Support
An increasing number of countries are implementing comprehensive national elimination plans; hepatitis testing is progressively being incorporated into national health insurance coverage systems.
Existing Challenges
Suboptimal Diagnosis Rates
Global diagnosis rates remain critically below established targets. As of 2022, only 13% of chronic hepatitis B cases worldwide had received a diagnosis (2). Between 2015 and 2022, merely 36% of hepatitis C cases were identified globally (3), representing a substantial shortfall from the 2030 target of 90%. Low-income countries face particularly severe diagnostic capacity constraints, with polymerase chain reaction (PCR) testing available in only a limited number of healthcare facilities. Testing access remains inequitable: rural and marginalized populations encounter significant barriers to diagnostic services, with cost, geographic distance, and social stigmatization serving as primary obstacles.
Uneven Treatment Coverage
Treatment accessibility demonstrates stark geographic and economic disparities. While coverage rates remain relatively high in high-income countries, patients in low- and middle-income countries (LMICs) face substantial barriers to medication access. By the end of 2022, only 3% of chronic hepatitis B patients globally had received antiviral therapy. Similarly, just 20% of hepatitis C patients underwent curative treatment during 2015–2022. Primary barriers include prohibitive drug pricing, supply chain disruptions, and insufficient capacity within primary healthcare delivery systems.
Inadequate Adoption of Innovative Technologies
Novel diagnostic tools demonstrate limited penetration at the primary care level. Portable technologies such as POCT and dried blood spot (DBS) sampling remain poorly integrated into national procurement systems, with healthcare facilities continuing to rely on traditional laboratory-based approaches. Digital follow-up systems show inadequate coverage: Long-term patient management frequently depends on paper-based records, resulting in elevated loss-to-follow-up rates. Artificial intelligence (AI)-assisted screening tools have not been incorporated into primary healthcare systems. Technology deployment faces significant barriers: Constraints in electrical infrastructure, internet connectivity, and training resources impede the implementation of innovative technologies in resource-limited settings.
Recommendations for the Future
The theme for China’s 2025 World Hepatitis Day — “Societal Co-governance for Hepatitis Elimination” — addresses core challenges through targeted solutions: 1) Diagnosis-Treatment Gap: Establishing an integrated ‘Screening-Diagnosis-Treatment-Management’ system through efficient screening protocols and tiered clinical management. 2) Immunization Protection Gap: Implementing precision interventions targeting high-risk adult populations. 3) Innovative Therapy Access: Accelerating functional cure protocols by expediting novel drug approvals and establishing clinical cure clinic networks. 4) Stigma Elimination & Social Mobilization: Advancing the ‘co-governance’ paradigm through multi-stakeholder engagement. 5) Policy Safeguards: Integrating hepatitis B virus (HBV) innovative therapies and hepatocellular carcinoma (HCC) early detection into national insurance coverage, establishing unified electronic hepatitis registries for real-time surveillance, and prioritizing grassroots resource allocation.
Aligned with the global 2025 theme “Hepatitis: Let’s Break It Down”, strategic recommendations emphasize dismantling structural barriers through 1) Innovative Screening Approaches: Establishing coordinated networks between community self-testing and centralized screening, promoting community self-testing technologies, optimizing centralized screening processes, and implementing a “three-step screening” collaborative mechanism (community initial screening, institutional fine screening, and hierarchical management); 2) Optimized Treatment Strategies: Advancing from pan-genotypic drugs to hepatitis B cure, optimizing pan-genotypic DAAs for hepatitis C research and application, and developing innovative functional cure protocols for hepatitis B; 3) Improved Resource Allocation: Constructing distribution systems for regional collaboration and international cooperation, establishing mechanisms for regional drug reserves and sharing, and innovating international cooperation through expanded drug patent pools, North–South cooperation production models, cross-border medical collaboration networks, and integrated diagnostic and treatment assistance; 4) Enhanced Technological Innovation: Developing rapid diagnostic equipment suitable for primary care settings, including portable non-invasive diagnostic devices, microfluidic chips and biosensing technology for grassroots applications, and artificial intelligence-assisted diagnostic systems; 5) Robust Monitoring and Evaluation: Constructing real-time dynamic monitoring systems, establishing multidimensional effectiveness evaluation frameworks, implementing quality continuous improvement mechanisms through “evaluation-feedback-optimization” cycles, and ensuring close integration between monitoring, evaluation, and policy adjustments.