The “hygiene hypothesis” proposes that reduced exposure to microbial infections in early life, especially in Western societies, contributes to the rise of allergies and autoimmune diseases [20]. In line with this hypothesis, emerging research indicates that helminth exposure may offer therapeutic benefits by promoting balanced immune responses. Although helminths elicit immune responses in their hosts, they also employ immune evasion mechanisms to avoid complete host immunity [21, 22]. Foxp3+ T regulatory cells play a crucial role in suppressing inflammation and maintaining tissue homeostasis [23]. Recent studies have shown that infection with parasitic helminths, such as Echinococcus multilocularis (Emu) [24], Heligmosomoides polygyrus [25], Hymenolepis diminuta [26], and so on, reduces chemically-induced colitis in mice, likely by modulating the host’s immune responses [27]. Consistent with these findings, our results revealed maternal Emu infection significantly alleviates colitis, as indicated by decreased weight loss, reduced DAI scores, and improved histopathological outcomes. Interestingly, emerging evidence suggests that Foxp3 expression is not limited to T cells. For example, in certain disease contexts, Foxp3 expression has also been identified in myeloid cells, including macrophages. Notably, Foxp3+ macrophages have been implicated in regulating poststroke neuroinflammation [28]. In our study, the observed elevated Foxp3 expression further supports the notion that Emu infection induces a robust immune regulatory response, which contributes to the reduction of colonic inflammation. Overall, these results align with previous studies suggesting that helminth infections modulate immune responses to protect against inflammatory diseases, such as IBD.
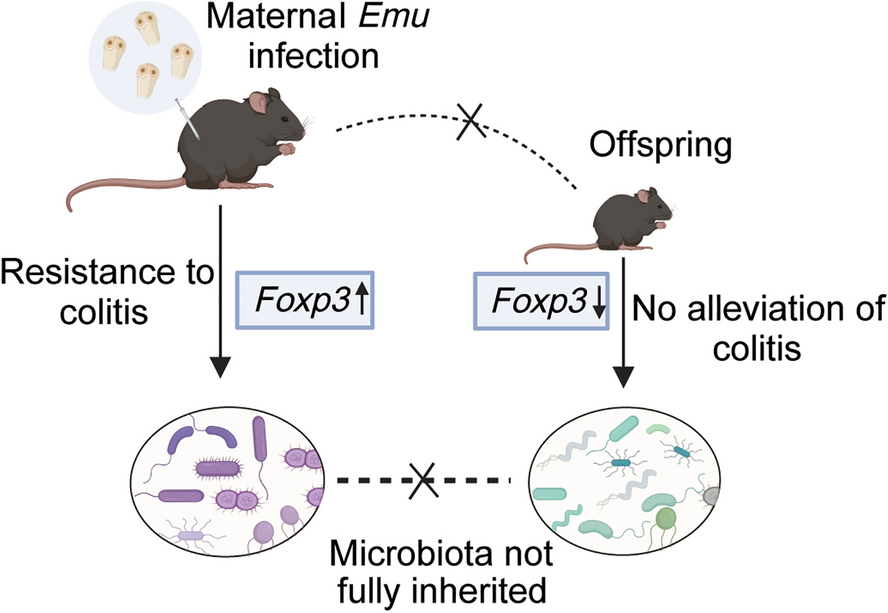
Maternal perinatal programming is a critical period of developmental plasticity, where environmental factors such as infections can have lasting effects on offspring health [29]. Previous studies reported that offspring of helminth-infected dams developed enhanced immunity against gut infections and altered inflammatory responses [30]. However, when we examined the potential inheritance of the colitis-resistance phenotype in offspring, our results showed that the offspring of the Emu-infected maternal mice did not exhibit similar ameliorative effects when subjected to DSS-induced colitis. Both offspring of the infected and control group demonstrated significant weight loss, high DAI scores, and elevated histopathological scores without notable differences. In addition, the absence of significant differences in Foxp3 expression levels in the offspring further supports the lack of heritability of the mother’s ameliorative phenotype. Various factors, including genetics, gut microbiota, diet, and environmental conditions could influence these findings.
The gut microbiota is crucial for host health, influencing immune function, metabolism, and behavior [31, 32]. Gut microbiota is largely shaped by early life environmental factors, prompting investigations into whether helminth infections affect not only the host but also their offspring [33]. The gut microbiota analysis provides insights into the potential mechanisms behind these observations. Our study revealed that maternal infection with Emu induced significant alterations in gut microbiota of maternal mice compared with naive mice. Specifically, the abundance of several bacterial genera, such as Rikenella, Rikenellaceae_RC9_gut_group, Turicibacter, Odoribacter, and Parabacteroides, was increased in the Emu-infected mice. Conversely, the abundance of genera such as Lactobacillus, Staphylococcus, and Bifidobacterium was reduced following Emu infection. These results highlight the profound impact of maternal Emu infection on gut microbiota composition, which aligns with previous studies showing that helminths can modulate gut microbial communities, influencing immune responses and potentially shaping long-term health outcomes [18, 34]. Interestingly, while the maternal microbiota exhibited significant changes, gut microbiota structure of the offspring from Emu-infected mice displayed a distinctly different pattern. In the offspring from Emu-infected mice, Candidatus Saccharimonas, Desulfovibrio, Helicobacter, and Odoribacter were significantly more abundant, whereas genera such as Muribaculum and Clostridium_sensu_stricto_1 were significantly depleted. This suggests that, despite the maternal microbiota changes, the microbiota of offspring was markedly different and did not fully mirror the maternal microbial profile. These findings suggest that maternal infection-induced shifts in microbiota may not be fully transmitted to offspring, potentially owing to developmental or environmental factors influencing microbiota establishment in the offspring’s gut. Despite significant differences in the gut microbiota structures of maternal and offspring mice, several similar trends were noted in both infected group and offspring mice, such as the increase in Odoribacter and Rikenella. These trends indicated that maternal gut microbiota alterations could partially influence the offspring’s microbiota composition.
Our findings reveal both consistencies and discrepancies when compared with previously published studies on helminth-induced microbiota alterations. Specifically, our results align with prior studies showing increased Bacteroidota abundance in H. polygyrus-infected mice [10, 35]. However, the observed reduction in Lactobacillus in C57BL/6 mice contrasts with a study indicating that Emu infection increased the abundance of Lactobacillus in Balb/c mice [18]. The role of Lactobacillus in regulating immune responses is well-documented, particularly for its anti-inflammatory effects [36]. Our unpublished data from the lab indicate that such differences may be species-dependent and influenced by baseline gut microbiomes and the specific stages of helminth infection. This highlights the complexity of microbiota inheritance and its dependence on various factors beyond maternal infection alone, including the origin of the mice used in the study. This is supported by our subsequent unpublished data, which show that even when using mice with the same genetic background but obtained from different facilities, infection with Emu results in distinct gut microbial responses, especially in terms of Lactobacillus abundance. These findings strongly suggest that variations in baseline microbiota significantly influence the direction of microbial shifts following helminth infection. In addition, as shown in Fig. 4, we also observed differences in the baseline gut microbiota compositions between uninfected maternal and their offspring mice, further supporting the notion. These observations may help explain the discrepancies in the microbial shifts between maternal and offspring mice. E. multilocularis as a tissue-dwelling helminth has been extensively studied for its capacity to modulate host immune responses [37]. Recent studies have shown that E. multilocularis infection significantly alleviates DSS-induced colitis in mice by suppressing Th1/Th17-mediated inflammatory responses [24]. In addition, research has found that the helminth-derived protein can mimic the function of human transforming growth factor (TGF)-β, inducing the activation of regulatory T cells [7]. Our previous findings also indicated that a serine protease inhibitor secreted by E. multilocularis (Emu-serpin) has been shown to mitigate colonic inflammation by reducing tissue damage and lowering the levels of proinflammatory cytokines in the colon [16]. It is possible that the observed effects are due to the excretory-secretory products released by the Emu during infection. This mechanism could potentially explain the observed amelioration of colitis in maternal mice following Emu infection. In addition, changes in specific microbial taxa may be involved. Odoribacter belongs to the Bacteroidota phylum and is generally considered a short-chain fatty acid (SCFA)-producing genus. Several studies have reported that the abundance of Odoribacter, particularly Odoribacter splanchnicus, is often negatively correlated with colonic inflammation, implying a potential protective role in maintaining gut homeostasis [38, 39]. Although Odoribacter increased in maternal and offspring mice, only the maternal mice exhibited reduced colitis susceptibility. This suggests that the presence of Odoribacter alone may not be sufficient to confer protection. Conversely, the notable increase in Desulfovibrio in offspring, a genus linked to IBD pathology [40, 41], may exacerbate inflammation, counteracting any inherited benefits from maternal microbiota. These findings underscore the complexity of host–microbiota interactions.
Nevertheless, this study has several limitations that should be considered. The establishment of E. multilocularis infection in the peritoneal cavity was associated with reduced reproductive performance in infected dams, leading to a limited number of offspring available for subsequent analyses. This biological constraint may have affected the sample size and reduced the statistical power of offspring-related observations. In addition, although the results indicate a potential association among Foxp3 upregulation, microbiota alterations, and reduced colitis severity in maternal mice, the precise molecular and cellular mechanisms mediating these effects remain incompletely understood. We need to include more makers and clarify the potential mechanism involved.