This hospital-based, cross-sectional observational study, despite its small sample size, aimed to evaluate the spectrum of RA in older patients and its subtype. This study reported an average age of 63.67 years with a high female-to-male ratio of 4:1 in the RA cohort, while in the OA cohort, the mean age was 65.5 years with a low female-to-male ratio of 2:1 overall. And, the duration of illness was 7.97 and 7.42 in RA and OA respectively. The reference to Chua’s study further supports these demographic differences, indicating that the mean age for OA patients was 66.1 years (± 10.6). In contrast, the RA patients on prior DMARDs had a younger mean age of 54.2 years (± 16.3), while those who were DMARD-naïve had an even lower mean age of 51.9 years (± 12.2). The median duration of disease was also notably different across these groups, with durations of 3.4 years for OA, 3.2 years for RA patients on DMARDs, and 1.0 year for DMARD-naïve RA patients. These disease duration differences in OA group could be due to its milder and more common nature, which often results in later-onset and longer-lived cases. The likely age divergence in RA group could be due to a selection bias favoring the recruitment of more severe and chronic cases, given the study’s setting in a tertiary care center [46].
The most prevalent RA symptoms included small joint pain and stiffness, along with constitutional symptoms such as fatigue, myalgia, fever, weight loss, rheumatoid nodules, lymphadenopathy, and neuropathy, consistent with previous studies. Contrary to the classical presentation of symmetrical small joint involvement in RA, 31% of older patients also exhibited larger joint involvement. Additionally, PMR-like symptoms were observed in approximately 19% of older RA patients, similar to findings in other studies [47, 48]. The most commonly associated comorbid conditions with RA included diabetes mellitus (19%), hypertension (47%), osteoporosis (52%), anemia (20%), cardiorespiratory diseases, and depression, paralleling the literature [14, 49,50,51]. Hypothyroidism was present in 20% of RA patients, consistent with the study by Enas A. Elattara which reported hypothyroidism as the most common thyroid dysfunction, occurring in 24% of RA patients, followed by subclinical hypothyroidism in 4% of patients, and subclinical hyperthyroidism in 1.3% of patients [52].
With compared to OA patients, comorbidities showed higher incidences of anemia, ILD, and osteoporosis (p < 0.05) in RA cohort. Conversely, OA patients had a higher prevalence of hypertension (p = 0.012). IADL impairment was notably higher in RA patients compared to OA patients (73.0% vs. 54.2%, p = 0.013), highlighting the substantial disability and reduced functionality typical of RA as a chronic, destructive illness of small joints. Other geriatric syndrome like nutritional status, cognitive function, and depression levels showed no significant differences, suggesting that RA may not inherently increase the risk of cognitive decline or depression compared to OA. However, a larger study may be needed to confirm these findings. Logistic regression analysis identified key predictors distinguishing RA from OA, including EMS (OR: 1.09, p < 0.001), fever (OR: 6.68, p < 0.001), weight loss (OR: 6.35, p < 0.001), myalgia (OR: 5.07, p < 0.001), osteoporosis (OR: 2.21, p = 0.013), ACCP levels (OR: 1.28, p < 0.001), and the novel marker 14-3-3η (OR: 2.87, p = 0.047). These factors further differentiate RA from OA, highlighting their significance in the disease pathology, as supported by multiple studies distinguishing between RA and OA.
Morning stiffness, a key indicator of disease activity, had a mean (SD) of 28.8(15.8) minutes, reflecting high disease activity at the time of recruitment. The mean (SD) TJC and DAS28 was 8.6(4.8), and 6.12(0.45) respectively, signifying high disease activity and poor improvement from baseline, suggesting inadequate medication control or adherence. The majority of patients were not in clinical remission. However, a possible selection bias towards recruiting more severe RA cases cannot be ruled out, as all patients were recruited from a tertiary care setting [11, 12, 29,30,31]. Classical rheumatoid hand deformities were observed in more than 20% of patients, with a Deformity Index (DI) Mean (SD), of 1.56(0.39), indicating significant disability in older Indian RA patients. The total SENS) mean (SD) on bilateral hand X-rays was 13.8(15.30) reflecting severe hand joint deformities, indicative of poor chronic disease control and ongoing joint erosion in older RA subjects [36, 37]. Regarding RA treatment, methotrexate (96%) and sulfasalazine (92%) were the most frequently used DMARDs due to their favorable cost-benefit ratio. In older RA patients, the use of biologic therapies and combination DMARD therapies was less frequent, likely due to the high prevalence of comorbidities and financial constraints. The most commonly prescribed painkillers were NSAIDs (55%) with PPIs (63%), as opposed to opioids (3%), due to NSAIDs’ anti-inflammatory properties and fewer sedative and emetic side effects. Over 80% of RA patients were on calcium and Vitamin D3 supplementation for osteoporosis prevention [9, 12, 32,33,34, 48].
Additionally, this study one step further attempted to classify and compared RA patients into two groups: YORA and EORA. The patients in EORA group were significantly older (mean age 67.87 vs. 62.23, p < 0.001) and had a later disease onset (mean age 64.68 vs. 52.68, p < 0.001). The duration in EORA was 3.1 years (± 2.5), significantly shorter compared to YORA, which had a duration of 9.55 years (± 5.77), with a p-value of < 0.001.The female-to-male ratio in the YORA and EORA group were 6.97:1 and 1.66:1 respectively. This group also showed a higher male prevalence (37.5% vs. 12.8%, p = 0.030), suggesting gender differences in RA manifestation as age advances. By comparison, the Turkcapar study reported that the mean age of patients with EORA was 71.7 ± 5.9 years, while for those with YORA, it was 52.1 ± 11.5 years. The female-to-male ratio was 1.48 for EORA and 2.85 for YORA (p = 0.012). Additionally, the average age of disease onset was 42.2 ± 10.4 years for YORA and 68.4 ± 4.6 years for EORA [14]. In our study, patients with EORA had a significantly higher age and shorter disease duration compared to those with YORA like Targonska-Stepniak study. However, unlike the study, where the EORA group included a higher proportion of men than the YORA group (25.4% vs. 8%), both groups in our study were predominantly female [11].
The classical clinical manifestations were similar in both groups, possibly due to the small sample size. EORA patients presented more frequently with constitutional symptoms such as fatigue, myalgia, fever, and lymphadenopathy, consistent with prior literature [53]. PMR-like symptoms were more prevalent in the EORA group (31.25%) compared to the YORA group (14.89%), this finding aligns with other studies [54]. Hypertension and ILD were more common in the EORA group, while DM, Sjögren’s syndrome, Felty’s syndrome, osteoporosis, and anemia were more frequent in the YORA group, likely due to the small sample size and the longer disease duration [11]. The prevalence of CAD was significantly higher in the EORA group compared to the YORA group, at 18.7% versus 2.1%, with a p-value of 0.047 suggestive inflammatory risk factors as CVD risk, especially in elderly patients [11, 15, 55]. Hypothyroidism was more frequent in the EORA group (31% vs. 17%), though this difference was not statistically significant, echoing findings from other studies [52].
Sulfasalazine was more commonly prescribed in EORA (100%) than YORA (89%) patients, while methotrexate (MTX) and hydroxychloroquine (HCQ) were more frequently used in YORA patients due to the higher side effect profiles and management difficulties in older patients with comorbidities. NSAIDS were used higher 61% in YORA group than 37% in EORA group, because of the higher risk of adverse events in older age group [32]. The study found that HAQ and DI scores were elevated in the YORA group, with a statistically significant difference indicated by a p-value of < 0.05. This suggests that patients in the YORA group experienced a more severe and disabling disease progression. These results may be explained by the longer disease duration observed in YORA patients [31]. Contrary to previous studies, this study did not find significant differences between YORA and EORA groups in the levels of ESR, CRP, hemoglobin, 14-3-3η and ACCP. This discrepancy may be due to the small sample size [11, 28].
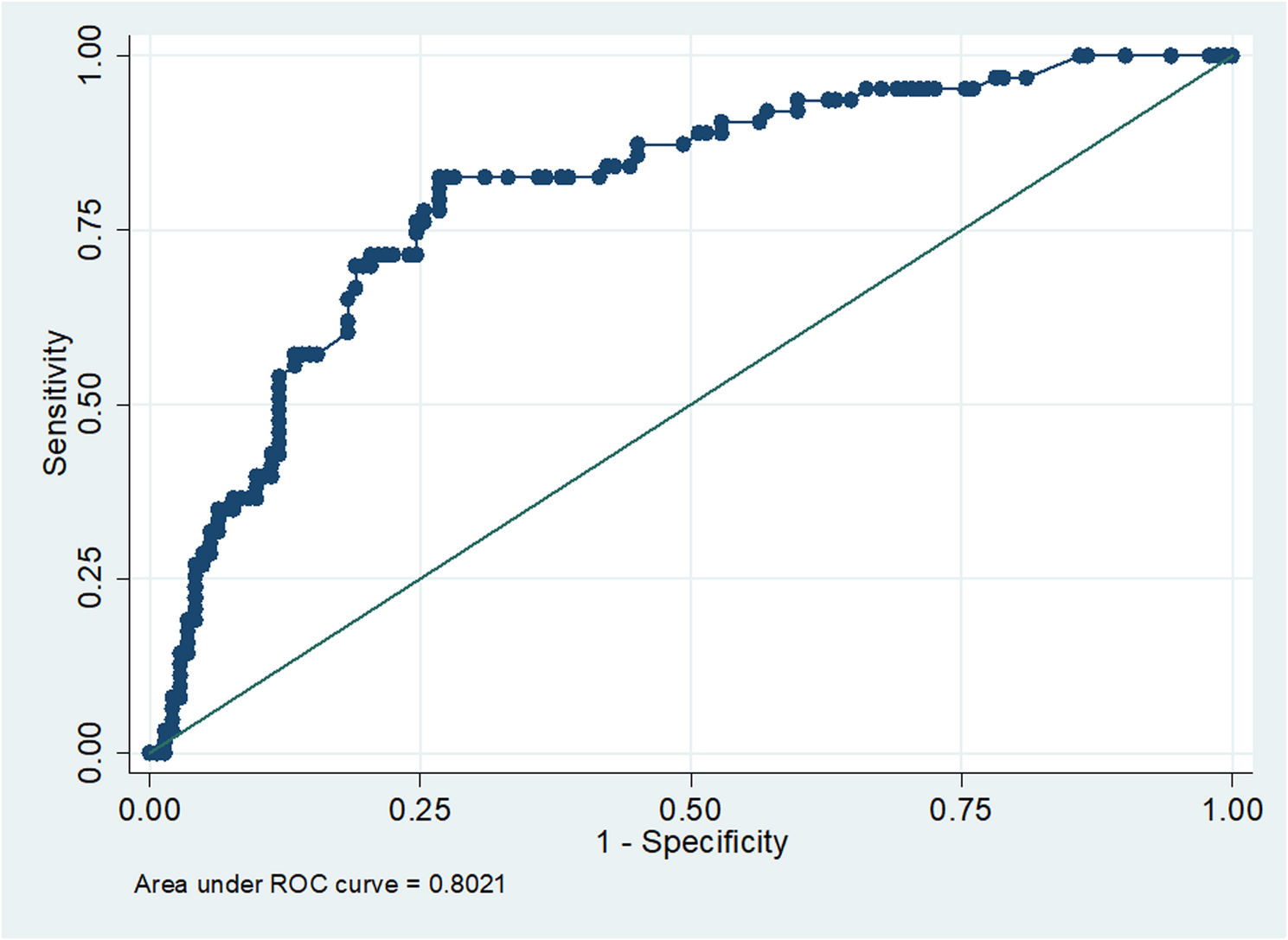
As explained in ROC curve, the cut-off value for serum 14-3-3η in detecting RA was higher than previous studies, with lower sensitivity and specificity, possibly due to the small sample size, unequal group numbers, prolonged disease duration, interference from methotrexate treatment, or variations in ELISA kit brands. The cut-off value for serum ACCP in detecting RA was lower than previous studies, likely due to differences in ELISA kit brands. However, the sensitivity and specificity at that cut-off were consistent with other studies [27]. Although the 14-3-3η protein has the potential to serve as a valuable complementary diagnostic marker, aiding clinicians in more effectively identifying high-risk patients, further investigation through larger studies is needed to gain a deeper clinical understanding of this novel marker.